The European Commission today launched the European Health Data Space (EHDS), one of the building blocks of a robust European Health Union. EHDS will allow the EU to take a huge leap 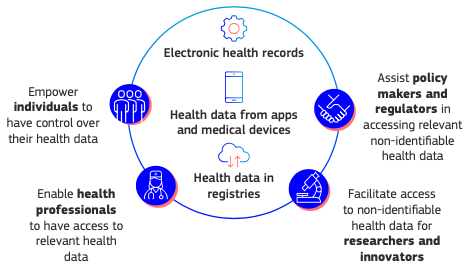 quality in the way healthcare is delivered across Europe: it will allow people to control and use their health data both in their own country and in other Member States, it will promote a true single market for digital health services and products, and will provide a coherent, reliable and efficient regulatory framework for the use of health data in research, innovation, policy-making and regulation, while fully complying with high EU data protection standards.
quality in the way healthcare is delivered across Europe: it will allow people to control and use their health data both in their own country and in other Member States, it will promote a true single market for digital health services and products, and will provide a coherent, reliable and efficient regulatory framework for the use of health data in research, innovation, policy-making and regulation, while fully complying with high EU data protection standards.
European Commission Vice-President Margaritis Schinas he has declared: “I am proud to announce the first common European data space in a specific sector. The European Health Data Area will be a 'fresh start' for EU digital health policy, putting health data at the service of citizens and science. Today we are laying the foundations for safe and reliable access to health data in full respect of the fundamental values on which the EU is founded.”
 Commissioner for Health and Food Safety Stella Kyriakides he has declared: “Today we are erecting another pillar of the European Health Union. Our vision is becoming reality. The European Health Data Space is a key paradigm shift for the digital transformation of healthcare in the EU. It puts citizens at the heart of everything and will give them full control over their data, so they get better healthcare across the EU. With access protected by strong security and privacy safeguards, the data will be a treasure trove for scientists, innovators and policy makers working on the next life-saving therapy. The EU takes a truly historic step towards digital health.”
Commissioner for Health and Food Safety Stella Kyriakides he has declared: “Today we are erecting another pillar of the European Health Union. Our vision is becoming reality. The European Health Data Space is a key paradigm shift for the digital transformation of healthcare in the EU. It puts citizens at the heart of everything and will give them full control over their data, so they get better healthcare across the EU. With access protected by strong security and privacy safeguards, the data will be a treasure trove for scientists, innovators and policy makers working on the next life-saving therapy. The EU takes a truly historic step towards digital health.”
Give citizens control over their health data both at home and abroad
- Thanks to EHDS it will be possible log into immediately and your data easily in electronic format, free of charge. It will be easy share such data with other health professionals even if they are in another Member State, with a strong improvement in health care. Citizens will have full control over their data and will be able to add information, correct incorrect data, limit access by third parties and receive information on how their data is used and for purposes.
- Member States will ensure that medical histories, electronic prescriptions, diagnostic imaging reports and supporting materials, laboratory reports and discharge notes are issued and accepted in a common European format.
- Interoperability and security will become essential obligations. Manufacturers of electronic systems for health records will need to certify that they meet these standards.
- To ensure that citizens' rights are protected, all Member States must appoint digital health authorities. These authorities will participate in the cross-border digital infrastructure (MyHealth@EU) that will help patients share data across borders.
Improve the use of health data in research, innovation and policy making
 The EHDS creates a solid legal framework for theusage of health data in research, innovation, public health, policy making and regulation. Under strict conditions, researchers, innovators, public institutions and the industry will have access to large amounts of high-quality health data, crucial for developing life-saving therapies, vaccines or medical devices and for ensuring better access to health care and more resilient health systems.
The EHDS creates a solid legal framework for theusage of health data in research, innovation, public health, policy making and regulation. Under strict conditions, researchers, innovators, public institutions and the industry will have access to large amounts of high-quality health data, crucial for developing life-saving therapies, vaccines or medical devices and for ensuring better access to health care and more resilient health systems.- In order to access the data, researchers, companies or institutions will have to ask for an authorisation to a body responsible for access to health data; these bodies will be set up in all Member States. Access will be granted only if the data requested are intended for specific purposes, in closed and secure environments and without revealing the identity of individuals. There is also a strict ban on using data to make decisions that could harm citizens, such as designing harmful products or services or increasing an insurance premium.
- The bodies responsible for accessing health data will be linked to the new decentralized EU infrastructure for secondary use of health data (HealthData@EU) which will be created to support cross-border projects.
Context
The COVID-19 pandemic has clearly demonstrated the importance of digital services in the healthcare sector. The adoption of digital tools has increased significantly during this period. However, the complexity of rules, structures and processes in different Member States complicates accessing and sharing health data, especially across borders. Furthermore, healthcare systems are also now increasingly targeted by cyber-attacks.
The EHDS builds on what has been achieved by the GDPR, by the proposal for a data governance act, from the draft data law and from directive on the security of network and information systems. It complements the initiatives mentioned and equips the health sector with more tailor-made rules. A open public consultation on EHDS took place from 3 May to 26 July 2021 and gathered a wide range of opinions, which were taken into account in the development of this legal framework.
The EHDS will also use the current and future productivity of digital public goods in the EU, such as artificial intelligence, high-performance computing, the cloud and intelligent middleware. The data space will also rely on the legal frameworks dedicated to AI, digital identity and cybersecurity.
Next steps
The proposal presented by the European Commission will now move on to discussion in the European Parliament and the Council.
For more information
Proposal for a regulation on the European Health Data Area
Data Strategy of 19 February 2020
The European health data space at the service of people and science
Source European Commission - Press release 3 May 2022
Related news: In Italy the new ones have recently been approved guidelines for the electronic health record.
Note:
 According to critics the pharmaceutical industry and multinationals will have access to the health data of individuals and with this detailed availability they will be able to have a decisive influence on how to design the health systems of the future. It will be anonymous data, it says, of course, but it is in the detail that the devil creeps in. Read the document. Sensitive data that should be highly protected, as written in the GDPR, the European Union regulation on the processing of personal data and privacy because we risk violating fundamental rights and freedoms, are sensitive data. But, as we have seen, with Covid, everything fell through.
According to critics the pharmaceutical industry and multinationals will have access to the health data of individuals and with this detailed availability they will be able to have a decisive influence on how to design the health systems of the future. It will be anonymous data, it says, of course, but it is in the detail that the devil creeps in. Read the document. Sensitive data that should be highly protected, as written in the GDPR, the European Union regulation on the processing of personal data and privacy because we risk violating fundamental rights and freedoms, are sensitive data. But, as we have seen, with Covid, everything fell through.
What use will industries make of this data? Who will actually analyze them? How long? With what purposes? Who will they sell them to? The EU document also states that "any attempt to use the data for possible measures harmful to the natural person, to increase insurance premiums, to advertise products or treatments, or to develop harmful products should be prohibited". Should. From the language it seems to be in the fairy tale of Little Red Riding Hood. In fact we have to ask ourselves, once this regulation has been passed, who guarantees a real defense of the citizen given the conflicts of interest between politics and pharmaceutical companies and the overflowing power of multinationals?
The proposal put forward by the European Commission will now be discussed by the Council and the European Parliament. It is the frontier that states and industry have been trying to cross for years, with the risk of further monstrate and dehumanize the society in which we live. And with a series of emergencies, such as Covid but also the war in the West, everything becomes easier, selling the fairy tale that the rights of individuals can be sacrificed to achieve goals that are more important to everyone. There is an emergency!!!
The business is served: from health data we move on to the data economy, an enormous and unstoppable flow of information that reveals sensitive data on citizens to make money for those who manage to manage it to sell goods. In this way, the companies that will enter institutions will be able to design the future. With artificial intelligence, the health budgets of the territories will be decided and, for those who have power and money in their hands, what is more convenient to do by redesigning the functioning of the social security funds, of the state health care, the budgets of the local health agencies and of the Regions (or decision-making bodies), deciding on what to invest and on what no, going into more and more detail, what should be treated and what not.
(Source: Italian affairs – 11 May 2022)