Pharmacovigilance
-
News

Farmacovigilanza. AIFA aderisce alla decima edizione della #MedSafetyWeek
AIFA aderisce alla decima edizione della #MedSafetyWeek, campagna di comunicazione internazionale per un uso più sicuro dei medicinali Comunicato AIFA…
Leggi » -
First floor

Ecofarmacovigilanza. Le aziende farmaceutiche chiamate a finanziare per almeno l’80% l’introduzione del trattamento quaternario nei depuratori
Ecofarmacovigilanza, l’impatto ambientale di farmaci si riversa nella direttiva Ue sulle acque reflue Le sostanze farmaceutiche vengono eliminate dal corpo…
Leggi » -
News

Aumentare la consapevolezza della farmacovigilanza sui social media. I doveri degli ISF
Aumentare la consapevolezza della farmacovigilanza sui social media Durante l’annuale #MedSafetyWeek, l’Uppsala Monitoring Centre (UMC) collabora con le autorità regolatorie…
Leggi » -
News

In Liguria progetto pilota per trovare sostituto al farmaco carente
Carenza farmaci: in Liguria progetto pilota per trovare sostituto al farmaco non disponibile Alisa ha costituito il comitato guida per…
Leggi » -
News

Supplements. Book: Herbs, foods and drugs. Between synergies and interactions
In the last twenty years, the scientific community has clearly brought the problem of the risks of interactions to everyone's attention…
Leggi » -
News

Liguria. A working group is set up with the aim of dealing with and managing information on drug shortages
Shortage of medicines, a pilot project for communication management in Liguria Alisa – 23 January 2023 Liguria before…
Leggi » -
News
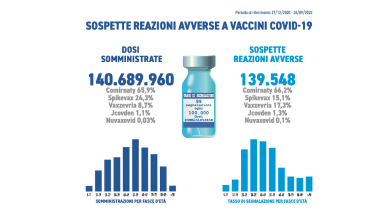
Report no. 13 of pharmacovigilance on anti-COVID-19 vaccines
The Italian Medicines Agency has published the thirteenth Pharmacovigilance Report on anti-COVID-19 vaccines. The data collected and analyzed concern…
Leggi » -
News
MedSafetyWeek 2022. Campaign on the safe use of medicines. The participation of AIFA
The Italian Medicines Agency (AIFA) is once again participating in the #MedSafetyWeek, the global campaign that aims to raise awareness among patients and operators…
Leggi » -
News

New warnings on the use of some drugs by EMA
The Safety Committee of the European Medicines Agency (PRAC) has published new warnings on the use of medicines containing terlipressin, ibrutinib…
Leggi » -
News

Clinical trials and innovative oncological drugs: does the sponsor influence the selectivity of a clinical trial?
Clinical trials of anticancer drugs are often criticized for their overly selective inclusion criteria. As we know, a…
Leggi »
