“Antibiotic resistance is a global problem, it is a real pandemic that has been going on for years and will continue to go on for many years. Italy is one of the countries that is paying the greatest price. Suffice it to say that almost 11,000 people die every year in Italy due to infections due to multi-resistant germs and this, evidently, gives the dimension of the problem and its impact not only in terms of deaths, but also at an economic and public health level " this is how Prof. Massimo Andreoni, scientific director of the infectious disease company SIMIT, defines the topic of antimicrobial resistance (AMR).
“The literature highlights how the correct approach to antibiotic resistance is an approach that tackles the problem in all areas; therefore from environmental sanitation, to people's behavior, to what is the control of antibiotic therapy, without doing without adequate risk control" continues Andreoni on the sidelines of the SIMIT 2021 Congress.
In recent years, the difficulty caused by the lack of new antibiotics in treating infections with multi-resistant germs has been repeatedly reported.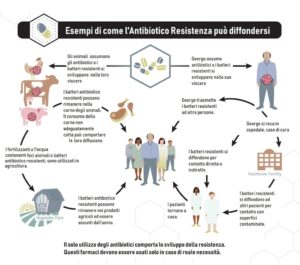
As AMR is a global threat, regulators in the EU, the US and Japan have agreed to align their data requirements as much as possible so that developers of medicines can design clinical trials that meet the testing needs of multiple regulatory agencies. The revised document reflects the outcome of these discussions and also includes:
- clarification of recommended clinical development schedules for antimicrobials intended to address an unmet need;
- clinical trial guidelines to support the treatment of uncomplicated urinary tract infections and uncomplicated gonorrhea;
- updated guidance on displaying microbiological and clinical efficacy data in the summary of product characteristics”.
The revised guidelines are published together with an addendum to guide the clinical development programs needed to support the authorization of medicines for the treatment of bacterial infections in children.
Source:
SIMIT -XX SIMIT Congress – Bacterial infections
Panorama of Healthcare – 26 May 2022
EMA – Guideline on the evaluation of medicinal products
indicated for treatment of bacterial infections -19 May 2022
EMA – Addendum to the guideline on the evaluation of medicinal
products indicated for the treatment of bacterial infections to
address pediatric-specific clinical data requirements – 19 May 2022
Related articles:
Fedaiisf – 18 Nov 2021 – European day on the conscious use of antibiotics