After the approval by the European Medicines Agency (EMA) of the Moderna vaccine, the Scientific Technical Commission of AIFA will meet on Thursday 7 January 2021 to examine the dossier on marketing authorization in Italy and define how to use it in the NHS.
 Moderna is a US company operating in the field of biotechnology. Founded in 2010, it is headquartered in Cambridge, Massachusetts
Moderna is a US company operating in the field of biotechnology. Founded in 2010, it is headquartered in Cambridge, Massachusetts
The company is active in the research and development of messenger RNA (mRNA)-based drugs.
On November 16, 2020, the company announced the discovery of mRNA-1273, an RNA vaccine to address the 2019-2021 COVID-19 pandemic, for which it claimed an efficacy of 94.5%, the 0, 5% less than the BioNTech and Pfizer vaccine, compared to which, however, it is easier to store.
The mRNA molecule is usually contained in a drug delivery medium, such as lipid nanoparticles, to protect the fragile mRNA strands and aid their entry into human cells. The fragility of the mRNA molecule requires cold chain distribution and low temperature storage, and may compromise effective efficacy due to inappropriate dosages. However, the Moderna vaccine remains stable at standard refrigeration temperatures between 2 and 8 degrees C for 30 days. In addition, long-term storage and transportation conditions are expected at standard freezer temperatures of -20 degrees C for 6 months
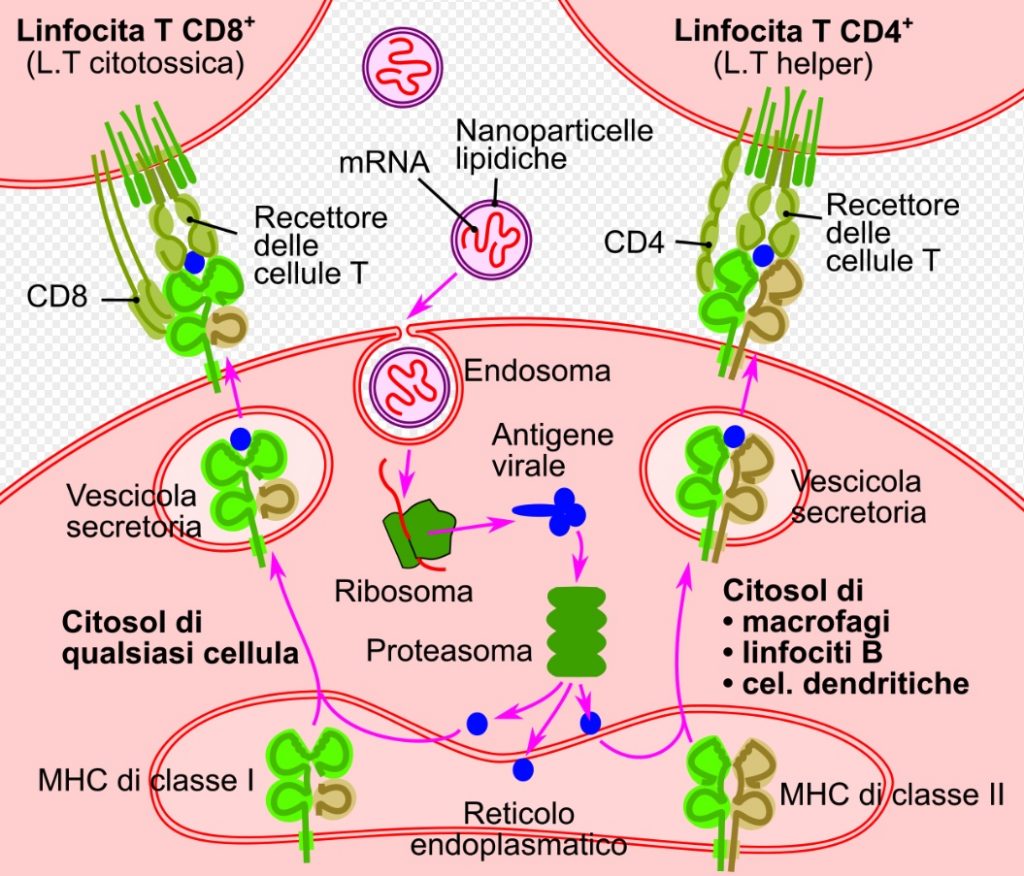 The technology used by Moderna for the vaccine consists of a compound of messenger RNA modified with nucleosides (modRNA), called mRNA-1273.
The technology used by Moderna for the vaccine consists of a compound of messenger RNA modified with nucleosides (modRNA), called mRNA-1273.
The mRNA-1273 delivery system utilizes a lipid nanoparticle drug delivery system.
Moderna relies on external companies and organizations operating under license to increase vaccine production: it has entered into a contract with Lonza Group for the production of the vaccine at the Portsmouth facilities in the USA and Visp, Switzerland. For the filling and packaging of the vials, Moderna has contracts with Catalent in the United States and Laboratorios Farmacéuticos Rovi in Spain.
On December 18, 2020, mRNA-1273 received emergency use clearance from the United States Food and Drug Administration. It was later authorized for use in Canada as of December 23, 2020.
At the end of 2020 Moderna obtained purchase agreements for its vaccine with the European Union for 160 million doses and with Canada for up to 56 million doses. Overall, 1.3 million Moderna doses will arrive in Italy in the next three months, on a weekly basis: 100,000 in January, 600,000 in February and 600,000 in March.
On 6 January 2021 it was authorized for sale in Europe by the European Medicines Agency.
Related news: EMA. COVID-19 Vaccine Modern
EMA recommends COVID-19 Vaccine Moderna for authorization in the EU
AIFA. Press release no. 623 [0.14Mb] >