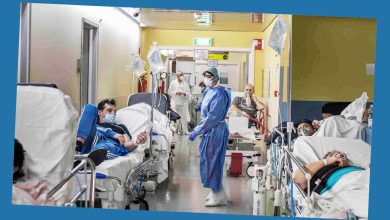
Ema says no to the use of AstraZeneca's Indian anti-Covid vaccine
"Covishield", produced with a similar technology but at a lower cost than the original AstraZeneca anti-Covid serum, "does not currently comply with EU standards".
 The European Medicines Agency has rejected the use of the anti-Covid serum "Covishield" produced by the Anglo-Swedish pharmaceutical company AstraZeneca in factories in India. The preparation is similar to Vaxzevria, authorized by the EMA for use in Europe.
The European Medicines Agency has rejected the use of the anti-Covid serum "Covishield" produced by the Anglo-Swedish pharmaceutical company AstraZeneca in factories in India. The preparation is similar to Vaxzevria, authorized by the EMA for use in Europe.
“Even if it uses a production technology similar to Vaxzevria (AstraZeneca's vaccine), Covishield does not currently comply with EU standards, – reads a note from the EMA, reported by Ansa. – This is because vaccines are biological products. Even small differences in production conditions can lead to differences in the final product, and EU law requires production sites and the manufacturing process to be assessed and approved as part of the permitting process."
The decision by the European regulator has raised concern in the African Union, since the Covishield is widely used.
We remind you that in the EU the EMA has approved 4 anti-Covid vaccines for the implementation of the vaccination campaign against the coronavirus:
- Pfizer-BioNTech (2 doses, manufactured by American and German company);
- Moderna (2 doses, produced by a US company);
- Johnson & Johnson (single-serve, manufactured by US company)
- AstraZeneca (2 doses, Anglo-Swedish company).
Just today Russian President Vladimir Putin he has declared to have been vaccinated against Covid-19 with the preparation of the Gamaleya Center.
Related news: AstraZeneca COVID-19 vaccine authorized for emergency use by the World Health Organization
Covishield: India puts pressure on EU for vaccine pass approval
Russia. 20,000 infections in one day: Putin sets an example and gets vaccinated