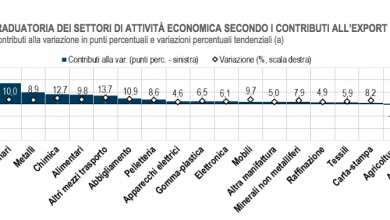
Brexit, the pharmaceutical industry raises the alarm: "Risks for patients in the EU and UK"
“Real, tangible and immediate threats to patient safety and public health, both in the UK and in Europe”. The prospect of the United Kingdom leaving the European Union without an agreement on the very near March 30, in a disorderly way, seriously worries Efpia, the federation that brings together the associations of drug manufacturers, which highlights the risks associated with the outcomes of the decision  of the British Parliament to reject the Brexit deal negotiated by the premier Theresa May (in the picture).
of the British Parliament to reject the Brexit deal negotiated by the premier Theresa May (in the picture).
The risks which, moreover, have already begun to materialize in the UK, where there is a rush to stock up on medicines. But patients from all over the Old Continent could be affected by a no-deal Brexit.
An article by EuropaToday, reporting the concerns expressed by the European Farminstria: “Now is the time for policy makers in the UK and EU to put politics aside and implement measures to prevent patients from being harmed by the consequences of Brexit." he claims Natalie Moll, CEO of Efpia. ” in in particular, from the interruption of the supply of medicines, including transport delays at the border, even when the development, manufacturing, packaging, safety testing and regulation of medicines no longer benefit from mutual recognition" .
The pharmaceutical industry claims to have worked "tirelessly" in these months to prepare to face the unexpected for each scenario. And he asks the negotiators to agree “a series of actions that must be taken to protect patients”. Starting with the introduction of measures to continue “to recognize tests made in the UK at least until they can be transferred to the EU; measures to enable the UK's continued participation in data-sharing platforms that protect public health and the safety of medicines in Europe; discussions between the competent authorities and the sector to coordinate contingency plans, such as the insertion of preferential lanes or priority routes for medicines in ports and airports".
 Not only: "Medicines and clinical trial materials – asks Efpia – should be temporarily exempted from new customs and border controls". It should also “allow paperwork and regulatory checks to be completed away from the physical border. And the European Aviation Safety Authority (EASA) should recognize UK-issued certificates to ensure planes can continue to fly.
Not only: "Medicines and clinical trial materials – asks Efpia – should be temporarily exempted from new customs and border controls". It should also “allow paperwork and regulatory checks to be completed away from the physical border. And the European Aviation Safety Authority (EASA) should recognize UK-issued certificates to ensure planes can continue to fly.
The European federation of drug industries is also asking the negotiators to "explore the possibility of also exempting active pharmaceutical ingredients (APIs) and medicine raw materials from border controls, to ensure that the production of medicines continues with limited interruptions".
For the sake of patients it is therefore necessary for policy makers to show “immediate and high attention to the issue of regulation and supply of medicines in the post-Brexit phase. We, along with many others in the healthcare community and across the life sciences industry,” concludes Efpia "We believe that an explicit commitment to ensure long-term cooperation on the regulation of medicines and medical technologies is in the interest of patients and public health."
Related news: EFPIA: Brexit
Hard Brexit will cost German companies 9 billion euros
Possible drug shortages after Brexit? Interview with the director of the European Medicines Agency