
Novartis has completely decoupled the price of the drug from the cost of its development, ensuring a 50% reduction on average current expenditure for treatment. The Zolgensma case born from the Telethon France offers and sold for 2.125 million dollars.
More News – September 29, 2019
There is certainly one very important thing that the first Conte government did, concluded a month ago, which was hardly talked about at all. A very courageous initiative, which the international press has covered a lot. With a concerted action by the Ministry of Health, in particular by the competent director  of the Italian Medicines Agency (AIFA), Luca Li Bassi, in 2018 Italy promoted a diplomatic obstacle course by presenting to the World Health Organization (who) a resolution with the aim of demanding transparency on the cost of medicines from the large pharmaceutical multinationals. Therefore, to ask for transparency on one of the best kept secrets in the industrial circles of the sector: the cost of research and development of new drugs.
of the Italian Medicines Agency (AIFA), Luca Li Bassi, in 2018 Italy promoted a diplomatic obstacle course by presenting to the World Health Organization (who) a resolution with the aim of demanding transparency on the cost of medicines from the large pharmaceutical multinationals. Therefore, to ask for transparency on one of the best kept secrets in the industrial circles of the sector: the cost of research and development of new drugs.
Access to medicines: no longer just a problem for the poor. Lack of access to essential medicines has historically been an issue affecting low-income countries. A battle for the right to health that is celebrating its twentieth anniversary, if we want to set its international debut for convenience with the mobilization of civil society - including doctors and patients - at the first interministerial conference of theWorld Trade Organization (WTO) in Seattle in November 1999. Ironically, but as was already foreseeable then, the problem has become global. Even rich countries have for some time been facing increasingly insurmountable obstacles, in terms of health budgets, to guarantee their citizens essential care.
 The most expensive drug in the world: 2.125 million dollars. The authorization for the sale in the United States of the most expensive drug in history, produced by the Swiss Novartis, dates back to May 2019. This is the drug Zolgensma, a genetic therapy that is administered with a single dose, and which is used for the pediatric treatment of children under the age of two suffering from spinal muscular atrophy (SMA). Zolgensma marks a historic step forward in the treatment of this rapidly developing disease; it is a revolutionary therapy because it is administered in a single dose. Its price is 2.125 million dollars.
The most expensive drug in the world: 2.125 million dollars. The authorization for the sale in the United States of the most expensive drug in history, produced by the Swiss Novartis, dates back to May 2019. This is the drug Zolgensma, a genetic therapy that is administered with a single dose, and which is used for the pediatric treatment of children under the age of two suffering from spinal muscular atrophy (SMA). Zolgensma marks a historic step forward in the treatment of this rapidly developing disease; it is a revolutionary therapy because it is administered in a single dose. Its price is 2.125 million dollars.
How much does research really cost? And yes: how much does research really cost to develop an innovative drug? Each case is unique, and recent genetic science has radically changed the scenarios of research in the pharmaceutical field, so it is not possible to give figures lightly. Since the battle over essential medicines has broken out, starting with antiretrovirals for millions of people affected by HIV/Aids in the southern hemisphere, the figures that estimate the cost of research have been wasted. They have grown from year to year. Often, blown up to become best-selling book titles (The $800 Million Pill, by Merrill Goozner) that revealed the captious narrative of the pharmaceutical industries. The most recent estimate is $2.6 billion.
The logic of profit is not in question. No one questions the corporate need to make profits, but medicines are goods of public utility that should not only be subject to commercial rules applied without discounts on a global scale, without even making the difference between essential medicines and therapies that are not essential. Vice versa, the rules established by the agreements on intellectual property of the WTO, which treat medicines like any other industrial product, give pharmaceutical companies an increasingly dominant position, because they operate in a regime of twenty-year patent monopolies.
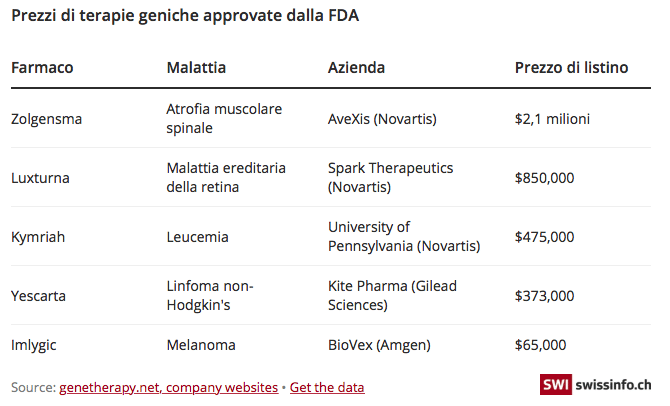 But let's go back to Zolgensma. Novartis said it built the cost of the drug on a “value-based pricing model” (value-based pricing model), ensuring a reduction of 50% on average current expenditure for the treatment of SMA, including the cost of a 10-year therapy for chronic SMA, around $4 million. The only alternative drug in use, Biogen's Spinraza, costs $750,000 for the first year, and $375,000 for subsequent years. While some financial analysts hint that the price set by Novartis could become a benchmark price for other gene therapies currently in development
But let's go back to Zolgensma. Novartis said it built the cost of the drug on a “value-based pricing model” (value-based pricing model), ensuring a reduction of 50% on average current expenditure for the treatment of SMA, including the cost of a 10-year therapy for chronic SMA, around $4 million. The only alternative drug in use, Biogen's Spinraza, costs $750,000 for the first year, and $375,000 for subsequent years. While some financial analysts hint that the price set by Novartis could become a benchmark price for other gene therapies currently in development
What Novartis doesn't say. Is that Zolgensma, whose sale it expects to make a profit of $2.4 billion a year, is the result of research initially funded by the marathon of Telethon in France. In this case, from a laboratory no profit created on purpose, Genethon, who has worked for years in the field of spinal muscular atrophy which paralyzes the muscles and respiratory system of children, with an investment of between 12 and 15 million Euros raised with the television marathon. The team of scientists had discovered that injecting a certain “viral vector” could correct the faulty gene. In March 2018, Genethon sold its patent to startups American AveXis, which already had Zolgensma in its research portfolio, for 15 million  dollars. The month after, AveXis was bought by giant Novartis for $8.7 billion. This means that Novartis has introduced in the American market, and will continue in the European and Japanese markets, a therapy that is the result of research financed by citizens' donations.
dollars. The month after, AveXis was bought by giant Novartis for $8.7 billion. This means that Novartis has introduced in the American market, and will continue in the European and Japanese markets, a therapy that is the result of research financed by citizens' donations.
Philosophy shapes the price of drugs. As Gilead Sciences had already successfully experimented, in 2013, with the innovative drug Sofosbuvir against hepatitis C (discovered by the biotech Pharmasset then acquired), launched in the USA at the prohibitive cost of 82,000 dollars for a 12-week therapy, and in Italy at a cost of 68,000 Euros, Novartis has completely separated the price of the drug from the cost of its development. If governments and international agencies such as the WHO accept the philosophy of basing the price of medicines on their intrinsic value, this will mean that life-saving medicines will end up costing more than others. And then yes, public health budgets will really be in danger.
Written by Nicoletta Dentico, an expert journalist on global health, he led the campaign to ban anti-personnel landmines and followed the campaign to cancel the debt of impoverished countries. Former director of Doctors Without Borders Italy
Note: Zolgensma is designed to address the genetic cause of SMA by delivering a functional copy of the human SMN gene to arrest disease progression through sustained SMN protein expression with a single intravenous (IV) infusion. Zolgensma is the first and only gene therapy approved by the FDA for the treatment of SMA, including those that are pre-symptomatic at diagnosis.
laria Giancaleoni Bartoli, Director of the Rare Disease Observatory (OMaR), wrote: Holder '2.1 million per dose' distorts the reader's opinion, because the dose is always and only one. Not to mention who called it 'the million dollar pill' by mistaking the type of drug – because it's not a pill but an infusion – and the price. Worse still, 'A single pill costs 1.2 million euros', even making the coin wrong, but thus insinuating the doubt that this price is paid in Europe, perhaps in Italy. All the Italian press headlined the price, the only element that does not apply to Italy: the therapy will be the same, the needs of the children the same, the price will not, that will be established by Aifa, our regulatory body, which knows how to do his bargains are very good. But flaunting the American price, owner of 'the most expensive drug', is accessed on social networks, and never mind if it doesn't do a service to readers.
Related news: Zolgensma for SMA: the most expensive drug in the world, 2.15 million dollars
Why can a drug cost $2.1 million?
SMA, how RNA targeted therapies have changed the history of the disease
Spinal muscular atrophy, the revolution underway: gene therapy is also coming
Zolgensma for SMA: the most expensive drug in the world, 2.15 million dollars
The criticisms primarily concern the way in which such a price is determined and the high profit margins of pharmaceutical companies, also taking into account the fact that very little is known about the development costs of these medicines. Novartis did not develop Zolgensma, but did own the drug through its $8.7 billion purchase of US company AveXis.
Many companies argue that prices are calculated using a value-based model, which raises a number of thorny questions about how much a life is worth. There is also a lack of evidence on the effectiveness of these treatments and the potential long-term risks. Some therapies that have come to market, such as Imlygic, have not lived up to expectations. Ethical questions related to gene manipulations also arise.
Ultimately, however, the main source of controversy concerns the burden on the community of these expensive treatments. Most health insurance schemes are not expected to take treatments at such prices. Novartis has previously said it is negotiating with insurers to allow payments over five years, at $425,000 a year, and to offer partial discounts if treatment doesn't work. But it is unclear how this will be implemented in each country. (swissinfo)





