The in-depth analysis on the future of life science professions continues with the manager who takes care of the transfer of productions from laboratory to plant or from one production site to another. Compared to the past, it has more skills and also comes from training courses not typical of the sector - From issue 157 of AboutPharma

Until a few years ago, the pharmaceutical tech transfer specialist was the person who managed the industrialization process of the molecules developed in the laboratory or who was in charge of transferring the production of a drug from one plant to another. Today, this figure (called, according to the hierarchies and the imagination of the companies, product tech transfer, tech transfer manager, etc.) is much more. He is a handyman profile, with transversal knowledge. He maintains relationships with other companies involved in the transfer, navigates through endless amounts of data and has a say in identifying technological innovations that can enhance the productivity of the company he works for.
The definition of tech transfer
To understand what the role is and how it has evolved recently, it may be helpful to start with a definition of technology transfer within the pharmaceutical industry. The quality technical committee of the American manufacturers' association Phrma (Pharmaceutical research and manufactures of America) defines technology transfer as "the body of knowledge available for a specific product or process, including critical product attributes in terms of quality, process, the 'capability' of the process, the manufacturing and process control technologies and the infrastructures of the quality systems”.
Types of technology transfer
The transfer can be of various types:
- from the laboratory to the plant that manufactures the products used in clinical trials;
- from the plant that produces batches for clinical trials to the site that industrially produces drugs for commercialization;
- from one industrial production site to another of the same company to balance production needs;
- outsourced: from a pharmaceutical company plant to a contractor plant;
- from a manufacturing plant to one where further testing, quality and safety checks and risk assessment are performed.
What does the tech transfer specialist in the pharmaceutical sector do?
The professional called to coordinate these procedures is precisely the tech transfer manager. First of all, it is he who is asked to express his opinion on the introduction of new products in a plant. Through feasibility analysis and an initial evaluation of transfer and production costs. Once the operation gets the green light, he takes care of setting up the team that will follow the transfer. 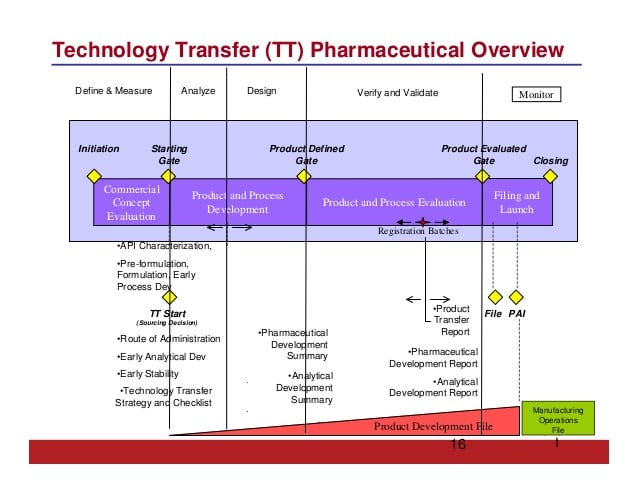 It then makes a forecast of the times within which this transition must take place, manages the budget assigned to the project and redistributes the tasks between the two "poles" of the transfer. If he is the specialist of a CMO, for example, he clarifies what the client company must do and what is instead the responsibility of the company functions. Always respecting the indications and quality standards required by customers and Gmp standards.
It then makes a forecast of the times within which this transition must take place, manages the budget assigned to the project and redistributes the tasks between the two "poles" of the transfer. If he is the specialist of a CMO, for example, he clarifies what the client company must do and what is instead the responsibility of the company functions. Always respecting the indications and quality standards required by customers and Gmp standards.
Check documents and assess risks
The realization of the project begins with the analysis of the documentation made available by the "original" manufacturer. In fact, it is necessary that the producer receives as much information as possible. These are data relating to the drugs to be made and the materials involved, from the active ingredients to the excipients, to the production process already possibly implemented by the other plant, to the instruments used and to the possible critical issues that can affect the quality of the products. It is up to the tech transfer specialist and his team to manage the data transfer, follow the analytical development and carry out all the necessary steps to validate the production processes transferred to the plant in which he operates.
Where to find instructions
What guides the work of those who do tech transfer are above all the guidelines drawn up by the most important regulatory bodies (Europe, the United States and Japan) within joint initiatives such as the Ich, or the International Conference for the Harmonization of Technical Requirements for the registration of medicines for human use. Furthermore, in the case of drugs that have already obtained a marketing authorization, a valuable point of reference for those involved in transfers is the registration dossier.
Green light to engineers and industrial production experts
If you look at the job postings, you can see that the required background is often very technical. Often the basic requirement is a bachelor's or postgraduate degree in Pharmacy, Chemistry, Pharmaceutical Chemistry and Technology, and the like. But compared to the past – and this is one of the first evolutionary lines of the profession – there are often pharma companies that gladly welcome profiles from Engineering studies. Figures who, only later, specialize in topics that most closely concern the chemical-pharmaceutical sector.
This trend started because the technology transfer specialist, to date, is called to have a multidisciplinary approach. And to play a role similar to that of the project manager. In addition to technical and analytical knowledge, he must have good knowledge of the most advanced industrial production methods. And he must know how to relate actively and collaborate with all company functions. Also because the teams to be coordinated have components that come from different areas, from research and development to marketing.
Introducing technologies, not just transferring them
The more he knows how to move between the departments of a pharma company, the better he can respond to the task that has been increasingly required of him in recent years. That is, understanding and proposing innovative technologies. Starting with those of the Industry 4.0 universe. With the aim of increasing manufacturing efficiency, reducing costs, decreasing environmental impact and creating more stable products with higher quality and safety for the end user. That is, the patient.





