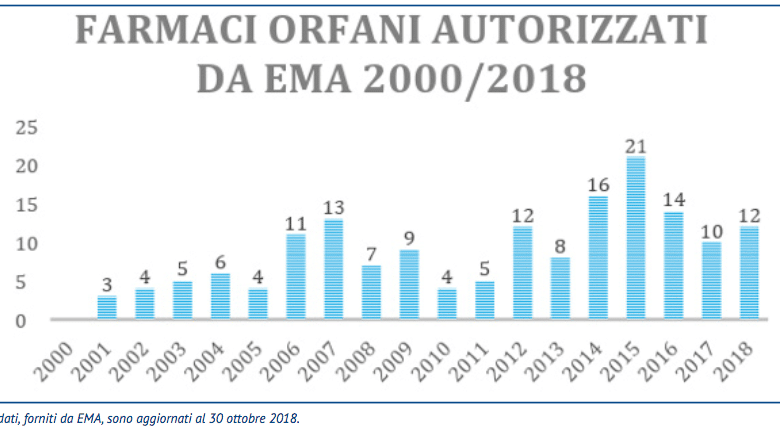
The government takes away 200 million euros a year from orphan drugs to give them to Big Pharma
A gift to multinationals and a heavy weight for small companies dealing with rare diseases. Moreover, without the state gaining anything.
AGI Health - 03 January 2019 by Ilaria Giancaleoni Bartoli
 Among the many concerns surrounding the Budget Law for 2019 one concerns rare diseases, and specifically the legislation to which Orphan Drugs are subject. In a nutshell, the government has decided to charge around 200 million euros each year to companies that are committed to rare disease patients by giving them away to Big Pharma and without even having an advantage for the state to offer as justification. The story is this.
Among the many concerns surrounding the Budget Law for 2019 one concerns rare diseases, and specifically the legislation to which Orphan Drugs are subject. In a nutshell, the government has decided to charge around 200 million euros each year to companies that are committed to rare disease patients by giving them away to Big Pharma and without even having an advantage for the state to offer as justification. The story is this.
It was introduced in 2012 to make ends meet for national pharmaceutical expenditure the payback mechanism: a ceiling on hospital expenditure is established if it is exceeded, all pharmaceutical companies must contribute to the level. It was realized that for rare diseases this represented a contradiction, as well as a strong disincentive to invest in an already difficult area: each additional orphan drug sold corresponded, by the very definition of an orphan drug, to a patient who, after years of waiting without a cure, a specific therapy could finally begin. It was not an expense that could be 'returned'.
Thus in 2013 the exemption was established for all orphan drugs from the application of the payback, an exemption also valid for orphan drugs which, although effective and intended for diseases previously without any therapies, had some 'defect of form', such as for example the 'having lost market exclusivity (it has happened, very rarely and for specific reasons, that two different drugs were put on the market for the same disease) or that they were “orphan-like” (approved before Regulation (EC) No. 141/2000 which introduced this definition and therefore could not have obtained it).
Orphans were thus considered a special category, to be protected and encouraged, not at the expense of the State: the 2013 law was conceived as a solidarity contribution by the largest pharmaceutical multinationals towards small and medium-sized biotech companies engaged in research in the sector . For these large companies, the impact was negligible, less than 1% of their annual revenue.
A gift to multinationals
With the latest budget law, however, both the principle that orphan drugs, all of them, are to be protected, and the principle of solidarity whereby big pharma helps companies involved in rare diseases have been swept away. In fact, it has been established that orphan likes and orphans who have lost exclusivity will return to pay, freeing Big Pharma from this small burden. There is talk of a total of around 200 million euros each year that are stolen from companies that deal with rare diseases, money that mainly ends up in research, but also in projects to help patients. 200 million euros that go back to the state coffers and which were used to bring back the difficult accounts of the Budget Law? No, 200 million going into Big Pharma's coffers.
A substantial but also symbolic and significant gift from the Government to the large drug multinationals: it is difficult to believe that a minister of the 5 Star Movement has done this, but the facts are in the text published in the Official Gazette. If the Government then decides to retrace its steps, as it seems it will do for the rule that penalizes volunteering, so be it: but of course the bad signal to rare patients has arrived loud and clear. Parliamentary questions and patient protests are expected in the next few hours, the Government will have to answer the first ones, we hope they will be listened to carefully.
Note: the payback portion attributable to orphan drugs was spread over non-orphan drugs
Related news: OSSFOR. Annual report