Press release no. 667 – The Italian Medicines Agency has published the ninth Report of 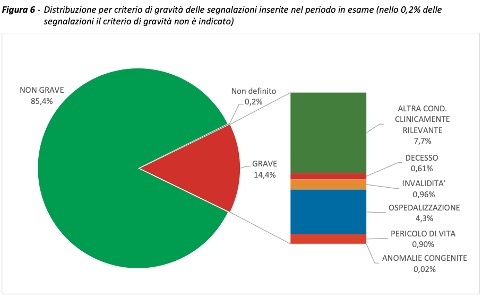 Pharmacovigilance on COVID-19 vaccines. The data collected and analyzed concern reports of suspected adverse reactions registered in the National Pharmacovigilance Network between 27 December 2020 and 26 September 2021 for the four vaccines in use in the current vaccination campaign.
Pharmacovigilance on COVID-19 vaccines. The data collected and analyzed concern reports of suspected adverse reactions registered in the National Pharmacovigilance Network between 27 December 2020 and 26 September 2021 for the four vaccines in use in the current vaccination campaign.
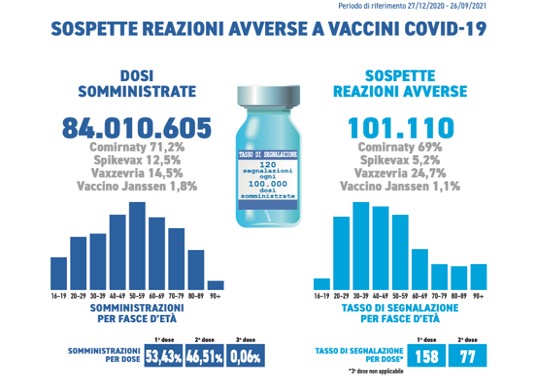
They were received during the period in question 101,110 reports out of a total of 84,010,605 of doses administered (report rate of 120 per 100,000 doses), of which the85.4% referring to non-serious eventssuch as injection site pain, fever, asthenia/fatigue, muscle aches.
Serious reports correspond to 14.4% of the total, with a rate of 17 serious events per 100,000 administered doses. As reported in previous Reports, regardless of the vaccine, dose and type of event, the reaction occurred in most cases (approximately 76%) on the same day of vaccination or the following day and only more rarely after 48 hours.
Comirnaty is the currently most used vaccine in the Italian vaccination campaign (71,2%), followed by Vaxzevria (14,5%), Spikevax (12,5%) and COVID-19 Vaccino Janssen (1,8%).
In line with previous Reports, the distribution of reports by type of vaccine follows that of administrations (Comirnaty 68%, Vaxzevria 22%, Spikevax 9%, COVID-19 vaccine Janssen 1%).
For all vaccines, the most commonly reported adverse events are fever, fatigue, headache, muscle/joint pain, local reaction or injection site pain, chills, and nausea.
In relation to so-called heterologous vaccinations people under 60 who received Vaxzevria as the first dose were received 262 reports, out of a total of 644,428 administrations (the second dose concerned Comirnaty in 76% cases and Spikevax in 24% cases), with a reporting rate of 40 for every 100,000 dosesadministered.
In the range of between the ages of 12 and 19, as of 26/09/2021 have been received 1,358 reports of suspected adverse event out of a total of 5,623,932 of doses administered, with a reporting rate of 24 adverse events per 100,000 doses administered. The distribution by type of adverse events is not substantially different from that observed for all other age groups.
With regards to the administration of third dose, which began in September, has only been carried out a report, compared to approx 46,000 doses administered.
Given the stability of the reporting trend for the various COVID-19 vaccines, the The surveillance report will no longer be published on a monthly basis but on a quarterly basis. On the other hand, the updating of the interactive graphs available on the AIFA website remains monthly.
AIFA – Published on: 12 October 2021PDF
Ninth AIFA Report on the surveillance of COVID-19 vaccines [3.71Mb] >





