Emilia-Romagna has over 130 thousand doses that must be used in the summer, but the administration of the fourth dose is very slow
 The regional councilor for health Raffaele Donini he said that in the hospitals and vaccination points of Emilia-Romagna there are 133 thousand doses of vaccine against the coronavirus due to expire in the coming weeks, between June and August. With the current trend of vaccinations, the risk of wasting them is high, but so far Emilia-Romagna has not received any indications on what to do with these doses. "They will have to be placed in the best condition in some way before they expire," Donini said. "They are engaged in regions that have lower numbers or in countries where vaccination is not at the level of Italy". Emilia-Romagna has over one million doses available: 746,000 doses of the Pfizer-BioNTech vaccine, 319,000 of pediatric Pfizer, 70,000 of Novavax and 20,000 of Johnson & Johnson (J&J).
The regional councilor for health Raffaele Donini he said that in the hospitals and vaccination points of Emilia-Romagna there are 133 thousand doses of vaccine against the coronavirus due to expire in the coming weeks, between June and August. With the current trend of vaccinations, the risk of wasting them is high, but so far Emilia-Romagna has not received any indications on what to do with these doses. "They will have to be placed in the best condition in some way before they expire," Donini said. "They are engaged in regions that have lower numbers or in countries where vaccination is not at the level of Italy". Emilia-Romagna has over one million doses available: 746,000 doses of the Pfizer-BioNTech vaccine, 319,000 of pediatric Pfizer, 70,000 of Novavax and 20,000 of Johnson & Johnson (J&J).
Emilia-Romagna is not the only region with this problem. The stocks of doses kept in the refrigerators of the vaccination points have accumulated due to the natural slowdown in the vaccination campaign due to the poor adherence to bookings for the third and especially the fourth dose.
Although the fourth dose is recommended for categories of people considered at risk, such as the elderly and the immunosuppressed, so far the percentage of people who have decided to receive it is quite low. In many regions, especially in the South, 4 percent of the audience identified by the health authorities was not exceeded. The region with the lowest percentage of adherence is Calabria, where only 2.9 percent of the people for whom it is recommended received the fourth dose.
Piedmont is the region with the highest percentage of adhesions, 22 percent, however in recent days it has been reported that many people over 80 have not shown up for the appointment set at the vaccination point. In a communicated, the Region explained that "more than 70% of the over 80s who received the appointment complied with it": therefore almost one in three people did not show up at the vaccination point.

Reservations for the fourth dose - called by the Ministry of Health "second booster", i.e. the second booster dose after the completion of the first vaccination cycle with two doses - were opened in early April. It is administered no earlier than 120 days after the third dose and people who are infected after the third dose are excluded.
The first people to receive it were the so-called frail, i.e. immunosuppressed people and people with other immune system problems. Even the immunosuppressed, like the elderly, can receive the vaccine again as long as a minimum interval of 120 days has passed since the first booster. Reservations were later opened to all senior citizens.
Faced with these data, the president of the Higher Health Council Franco Locatelli last week It reaffirmed the importance of promoting the fourth dose among people at risk. Locatelli spoke of the current pace of the vaccination campaign as a result of a «vaccine fatigue, which in some respects can be taken into account and even, if we like, understood. It is absolutely essential that there is broad adherence to the administration of the second booster dose both for those over eighty and for guests of residential facilities for the elderly, but also for the 60-79 age group with coexisting pathology.
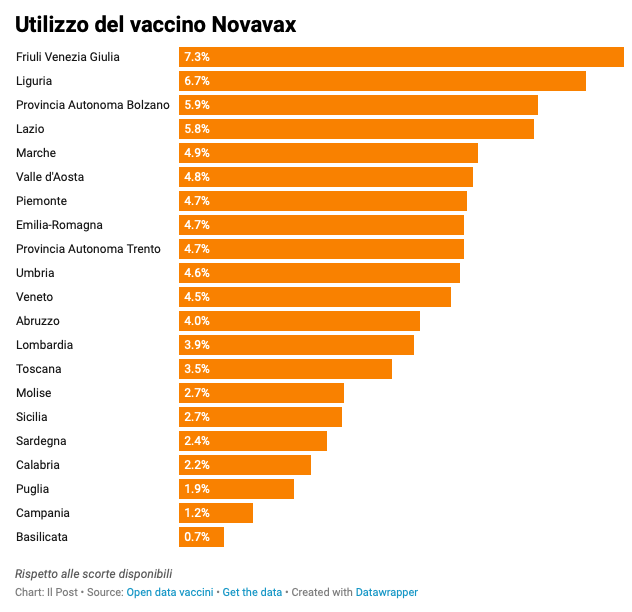
Among other things, the data published by the commissioner confirms that the accumulation of stocks mainly concerns some vaccine suppliers: Novavax and pediatric Pfizer.
Novavax is the first vaccine to use the technology of recombinant proteins and for this reason considered useful for convincing people who are skeptical of messenger RNA and viral vector vaccines. In the months in which the authorization of the European health authorities was awaited, there was a lot of expectation on this vaccine, which according to the most optimistic observers would have made it possible to achieve high protection against the coronavirus. In fact, already a few days after the opening of reservations it was clear that Novavax would only convince a fraction of the skeptics.
The result is that very few doses have been administered in all Italian regions so far, a very low percentage of the million doses delivered by the commissioner structure. A total of 40,000 doses were administered. In no region was the 10 percent utilization rate exceeded: the region with the highest percentage is Friuli Venezia Giulia, while Basilicata is the region where it was exploited the least.
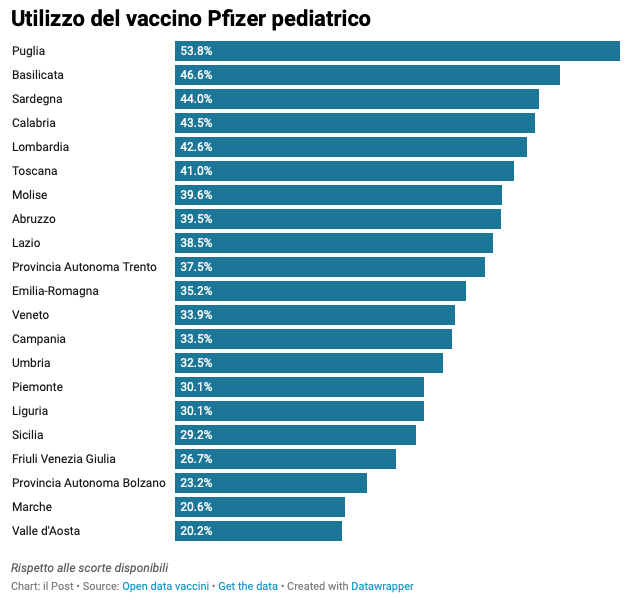
Another vaccine used little compared to forecasts is the so-called Pediatric Pfizer, recommended between 5 and 11 years: almost 2.6 million doses have been administered against the 6.7 million available. Also in this case the percentages of adhesion vary from region to region. The only region that has used more than half of the doses of pediatric Pfizer received in recent weeks is Puglia. The region with the lowest percentage of use is Valle d'Aosta.
The risk of expiration of thousands of doses has already been partially avoided in recent months thanks to a update of the leaflet of the Pfizer-BioNTech vaccine, the most used in Italy. On 21 September 2021, AIFA, the Italian Medicines Agency, had extended the duration of validity of the frozen vial with the vaccine inside from 6 to 9 months.
The three-month extension had also been applied retroactively to vials produced before September last year: the deadline had been extended by three months for all boxes bearing a date between September 2021 and March 2022, but only if stored according to indications, then at a temperature between -90 °C and -60 °C. All vials with an expiration date after March 2022 are already updated with the new 9 month shelf life.
The extension of the deadline, however, only concerns the Pfizer-BioNTech vaccine, not Novavax, Moderna or Johnson & Johnson. In the coming weeks, the regions will have to check how many doses they have accumulated in the vaccination points to carefully plan their use: they can be distributed from one province to another, depending on the demand and rate of vaccinations, or returned to the commissioner structure as had already happened last summer with AstraZeneca vaccines that the regions no longer wanted to use.
News correlate: Eleventh AIFA Report on the surveillance of anti-COVID-19 vaccines
Still written no vax on the walls of a CGIL headquarters in Reggio Emilia
Valneva vaccine, EU terminates purchase contract: why? / EMA authorization was missing





