Meetings between doctors and pharmaceutical informants: the Ospedale Maggiore of Parma sets new rules
Authorized and tracked appointments, in the afternoon and in dedicated venues. The hospital-university company implements the regional legislation
Press release AOU of Parma - 12/06/2017
Scientific representatives of drugs or medical devices who wish to meet the doctors of the Parma University Hospital must belong to thelist established in the Emilia Romagna Regionrequest express authorization from the Pharmacy and Clinical Government Service of the drug of the Company itself, show up for appointments with the identification card issued by the Region which then censuses the list, respect the established time slot and go to the company premises identified for this activity.
These are the new rules implemented by the Health Directorate of the Maggiore of Parma to regulate the access of scientific representatives within the hospital area with the aim of limiting direct contact between representatives and individual doctors for the presentation of drugs or devices. In the same way, information activities will no longer be allowed in outpatient clinics or in hospital wards, at any time of the day.
The meetings should preferably be collective and it will be the Company's task to promote them, but individual appointments must also be expressly authorized by the Pharmacy and Clinical Drug Administration service and organized by the director of the relevant department or operating unit.
Responsibility for organizing the event lies with the director of the relevant Department of the Structure concerned or the Professional object of the scientific dissemination activity by ISF at his or her own medical office, involving and informing the director or manager of the Structure concerned or his delegate.
While each request from the scientific representative must also indicate the name and specifications of the drug or medical device that you want to make known; the list of Structures proposed for the information activity, any free samples that will be delivered. The meetings can take place in the premises identified by the Health Department (about ten divided between the different areas in which the Parma Hospital is divided) or in the company medical offices, exclusively in a time slot between 3 and 6 pm. Scientific information activities will not be admitted outside the established prescriptions that fully implement the legislation established by regional resolution.
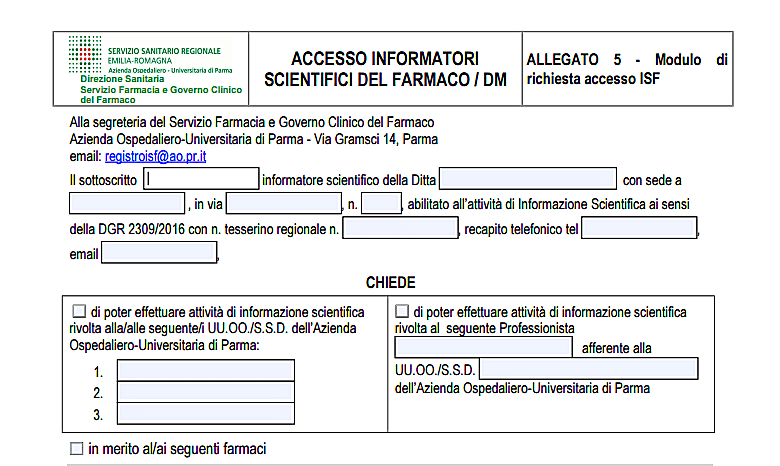
The module
Medical-scientific rep meetings:
L'organization of the event it is the responsibility of the Director of the relevant Department of the Structure concerned or of the Professional involved in the scientific dissemination activity at his or her own medical practice, involving and informing the director or manager of the Structure concerned.
Lscientific rep must submit a request at the Pharmacy and Clinical Drug Administration service (email: registryisf@ao.pr.it).
The meetings can take place in dedicated rooms (list) or in company doctors' offices, between 3 and 6 pm.
The request, duly completed and signed, it must be sent to the secretariat of the Pharmacy and Clinical Government Service of the drug, by email, at the address: registryisf@ao.pr.it
The Pharmacy Service and Clinical Drug Administration carries out, within 10 working days, an initial assessment of the adequacy of the request And will send the authorization only to the Director of the Department or the Head(s) of the Operating Units or Simple Departmental Structures concerned.
The Service will send the Scientific Informant the notification of receipt of the request email and any negative response. The Service Staff cannot release information regarding the status of the authorizations being processed.
 Anthony Balestrino, Medical Director of the AOU of Parma. On the site of the Parma Hospital is described as an expert”with specialization in monitoring and control of the appropriateness of services and diagnostic-therapeutic pathways“. After the shame of Prof. Fanelli's pain center scandal, he should resign for not having checked anything, or at least cancel certain statements that are just a joke. For information purposes, we inform Prof. Balestrino that Scientific Representatives have nothing to do with Prof. Fanelli.
Anthony Balestrino, Medical Director of the AOU of Parma. On the site of the Parma Hospital is described as an expert”with specialization in monitoring and control of the appropriateness of services and diagnostic-therapeutic pathways“. After the shame of Prof. Fanelli's pain center scandal, he should resign for not having checked anything, or at least cancel certain statements that are just a joke. For information purposes, we inform Prof. Balestrino that Scientific Representatives have nothing to do with Prof. Fanelli.
The meeting request form is simply mind boggling, all we need is the revenue stamp and the private habits of the ISF, if it were revised in a rational sense it would certainly earn its reputation. He should also know, given the role he covers, that from 3 to 6 pm, in most wards, the doctors who work the full day are no longer there and only those on call can be found. Find out what he says he's directing, don't make fun of us, don't make fun of himself!
We also inform the esteemed Prof. Balestrino that the representatives of dermocosmetics, baby milk, mineral and thermal waters, scientific encyclopaedias, teats, chamber pots, etc., etc., not being drug or device informants, will obviously have free access. A punitive discrimination against us and meaningless devices. More and more ridiculous!
And above all, find out about the laws and characteristics of the world it wants to "regulate". A world made up of professionals who, with scientific information, whether on drugs, nutraceuticals, supplements or medical devices, support the doctor in the most suitable and correct therapy for the patient, the only reason why the National Health Service exists. With this module, he forgets the ultimate goal of the existence of the healthcare facility he manages.
Ed
Related news:
Indications_applications_ISF – Reg. ER Annex 1