Six people had been hospitalized after taking an experimental molecule as part of a therapeutic trial programme.
17/01/2016 – tio.ch Ticino online
 RENNES – The volunteer who, hospitalized since January 7, had been in a state of brain death for two days in the university hospital of Rennes after undergoing a test for a new drug has died.
RENNES – The volunteer who, hospitalized since January 7, had been in a state of brain death for two days in the university hospital of Rennes after undergoing a test for a new drug has died.
The conditions of the other five patients who are under observation remain stable, of which four would have neurological problems and could risk permanent damage, while the fifth would not present particular symptoms but would be held as a precaution. All six are men and are between 28 and 49 years old. Investigations into the incident are underway by the judicial and health authorities.
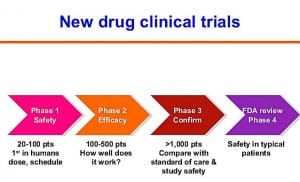
The test of the synthetic cannabinoid molecule tested as a pain reliever and to act on mood disorders was 'phase 1', i.e. in the initial development period, and was conducted by the French research center Biotrial on behalf of the Portuguese pharmaceutical company Bial. The oral experimentation began on 7 January and involved 90 'human guinea pigs', all volunteers, healthy and paid (just over a thousand euros for a week; the annual ceiling is 4,500, Biotrial specified).
After what happened, the hospital contacted the remaining 84 volunteers and yesterday ten underwent checks which did not reveal the clinical and radiological anomalies present in the hospitalized patients. The university center will coordinate the support of clinical centers to which some of these volunteers h asked to be followed up.
asked to be followed up.
On an episode that the director general of Biotrial defined as "unforeseen, unpredictable and inexplicable", also because the molecule had all the authorizations and the test was carried out "in full application of international regulations", three investigations are underway: one of judicial police, one from the general inspectorate of social affairs sent by the Ministry of Health and one from the national drug safety agency.
The molecule had first been tested on chimpanzees and since June 2015 it had passed to tests on humans, which involved a total of 108 patients, of which 90 took the molecule and the remainder a placebo. Only the six hospitalized, however, had taken the same dose of the drug several times and increasingly while the others had taken a single dose.
On Friday, the hospital's chief neuroscientist, Gilles Edan, said three of the patients were at risk of permanent brain damage.
Related news: France. Rennes: a clinical study ends badly. The test involved a painkiller
France, test on new drug ended in tragedy: even dogs were dead
A Biotral test volunteer earns between 100 and 4,500 euros. In Portugal, payment to anyone intending to participate in clinical trials is prohibited.
Guinea pig profession, 1,900 euros
How and why you can die if you are guinea pigs for drug testing