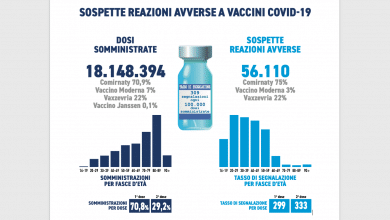
The drug Plaquenil is not in shortage today but the demand is so high that there are unavailability at wholesalers and pharmacies. This was reaffirmed by a Sanofi press release which highlights how the current request exceeds the 5 times the  historical average consumption with a constantly growing trend.
historical average consumption with a constantly growing trend.
At this juncture, in order to meet all the requests received, Sanofi requires significant support from healthcare professionals to rationalize the use of the medicine: reserving the use of PLAQUENIL only in cases where it is considered essential, to guarantee the therapeutic continuity; avoiding hoarding by virtue of the fact that the medicine is available only on medical prescription; avoiding stock accumulation; reminding those who prescribe Plaquenil for the treatment of COVID-19 patients to promptly transmit to the AIFA pre-authorization area the data relating to patients treated according to the methods indicated on the AIFA «COVID-19 Emergency» website, at the following address: https://www.aifa.gov.it/emergenza-covid-19, as required by the Resolution 17  March 2020.
March 2020.
PFor urgent needs, the press release invites healthcare professionals and all operators to refer to the Customer Service toll-free number at no. 800-103330 or to the e-mail address customerservice.farmacie@sanofi.com pTo receive instructions to place a dedicated direct order (according to the real needs of the patient) according to Legislative Decree 219 art. 105 paragraph 4.
Finally, Sanofi underlines that the quantities of medicine necessary for the treatment of COVID-19 patients will be available on the hospital channel and dispensed from hospital pharmacies, as per Det. AIFA no. DG 258 of 17.3.2020 published in the Official Journal no. 69 of 17.3.2020.
Sanofi. About hydroxychloroquine
April 6 update – In recent days we have recorded a significant increase in reports of a lack of hydroxychloroquine in pharmacies due to an ever-increasing demand for the drug and tension in the distribution chain. All of this is taking the form of temporary supply difficulties for wholesalers and pharmacies throughout the country.
 We are aware of the continuation of this situation which is also a source of great concern for us. In fact, although, to date, considering the therapeutic needs, we do not detect a shortage problem, we find a growing difficulty in satisfying all the requests since they have increased exponentially. In March alone, Sanofi distributed more than double the usual average quantities on the market (283,000 packs against the usual 130,000). In recent days, the average number of packages requested has exceeded 25,000 per day.
We are aware of the continuation of this situation which is also a source of great concern for us. In fact, although, to date, considering the therapeutic needs, we do not detect a shortage problem, we find a growing difficulty in satisfying all the requests since they have increased exponentially. In March alone, Sanofi distributed more than double the usual average quantities on the market (283,000 packs against the usual 130,000). In recent days, the average number of packages requested has exceeded 25,000 per day.
Globally, we have implemented additional sourcing efforts to meet growing product demand, increasing our manufacturing and distribution capabilities. Thanks to these efforts we are continuing to supply hospitals and pharmacies.
Despite the complex health emergency linked to COVID-191 we are at the forefront to ensure therapeutic continuity for patients suffering from rheumatoid arthritis and SLE (systemic lupus erythematosus) for which this drug is indicated. For them we are proceeding with an emergency distribution and shipment which is activated by the single pharmacy upon presentation of the medical prescription of the drug for chronic therapy.
At the same time we are cooperating with patient associations, doctors, pharmacists and institutions to ensure the availability of the drug, in an appropriate manner, to the people who need it, avoiding unnecessary hoarding phenomena as much as possible.
With the aim of ensuring maximum listening to patients and healthcare professionals, we are activating an additional service Specific Customer Service for this product dedicated to pharmacies and hospitals.
Furthermore, we have strengthened the existing call services, including that of Medical Information aimed at patients (tel. 800 536389 – information.medicalscientifiche@sanofi.com) so that we can provide all the necessary multi-channel support. These services can count on the support of colleagues from other company functions who have made themselves available to enhance the service and make it more efficient.
We invite those who find themselves in an extremely urgent situation and are unable to find the drug to rely on their doctor and pharmacist to find the most appropriate solution.
Sanofi also wishes to remind you that hydroxychloroquine is a drug that can be dispensed only and exclusively on medical prescription and under medical supervision, considering the possible adverse events. AIFA has authorized its reimbursement for the treatment of patients affected by COVID-19 under specific conditions: in addition to the diagnosis of COVID-19, the product must only be dispensed by hospital pharmacies which are required to promptly send the Italian Medicines Agency the data relating to patients treated with the medicine. AIFA also reiterated that prophylaxis use should only be considered in the context of clinical trials.
Based on the currently available data relating to the therapeutic needs both for rheumatic diseases and for the diagnosis of COVID-19, the availability of hydroxychloroquine for prescriptions in an appropriate regime is  ensured in sufficient quantities.
ensured in sufficient quantities.
In this situation of great health emergency, we appeal to everyone's responsibility to ensure the appropriate methods of using the drug, to avoid any form of "do-it-yourself" use of the drug, and to avoid unnecessary hoarding and family stockpiling practices.
1Sanofi specifies that the use of hydroxychloroquine in the treatment of patients with COVID-19 is to be considered off-indication (so-called off-label), as the preliminary data of several independent studies require further analyzes and larger and more robust clinical studies to be able to confirm the benefit/risk profile of hydroxychloroquine for this use.
In Italy, on 17 March the Technical-Scientific Commission of the Italian Medicines Agency (AIFA) had expressed a positive opinion on the use of hydroxychloroquine for the treatment of infections caused by the new SARS-CoV2 coronavirus. With the Official Gazette of March 17, hydroxychloroquine was authorized for the treatment of patients suffering from COVID-19 and included in the list of drugs paid by the National Health Service for this use. The product must be dispensed by hospital pharmacies. Furthermore, the prescribing structure is obliged to promptly transmit to AIFA the data relating to patients treated with the drug according to the methods indicated in the appropriate section of the AIFA institutional website «COVID-19 Emergency», at the following address: https://www.aifa.gov.it/emergenza-covid-19