We receive and publish:
We inform you of the start of the corporate project called STEM which provides for the imposition of the support by the consulting company Stem Healthcare to the ISFs with doctors during their daily scientific information activity.
This critical point did not find a negotiation resolution and led to the breaking off of the negotiations between the RSU of external personnel and the corporate management of Angelini Pharma with the consequent proclamation of a strike for Monday 15 May, an event that had never happened in the history of the external network of the Angelini Pharma.
We kindly ask you to communicate this protest initiative on your category communication masthead so that our event has the greatest media visibility.
Thanks for your availability, we will keep you updated on developments.
The RSU of Angelini Pharma's external personnel.
______________________
Editor's note:
 It is already relevant that a figure, that of the ISF, is required to carry out commercial activities, because that is the purpose of the STEM, which, by express provision of the law, is absolutely incompatible, having only to carry out scientific information.
It is already relevant that a figure, that of the ISF, is required to carry out commercial activities, because that is the purpose of the STEM, which, by express provision of the law, is absolutely incompatible, having only to carry out scientific information.
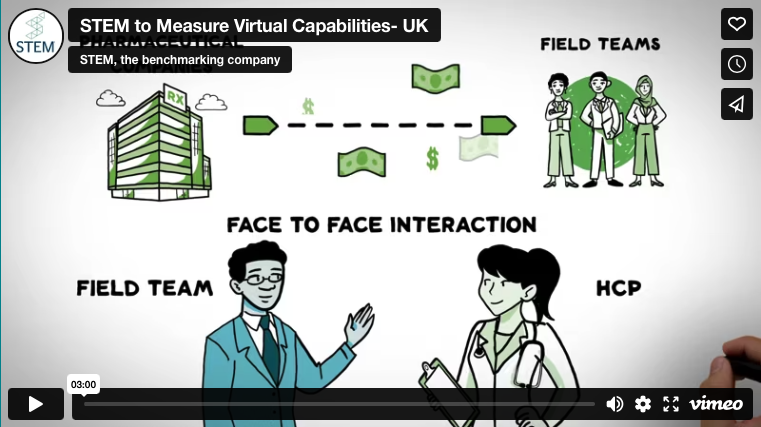
The activity of the Pharmaceutical Scientific Representative, and consequently scientific information, (art. 122 of Legislative Decree 219/06) can be provided to the doctor and pharmacist, in a limited form for the latter, by scientific representatives. Therefore it cannot be done in the presence of a stranger, such as the Stem official, as he is neither a doctor nor a titular or hospital pharmacist.
The ISF is part of a Scientific Service (art. 122 And 126 of Legislative Decree 219/06) on which it depends and the Scientific Service must be independent from the Marketing Service of the pharmaceutical company (art. 126).
The authentic interpretation then comes from AIFA with the letter from Dr. Magrini, then DG, of 23 March 2021, where he states: “the art. 122 indexed “Requirements and activities of scientific representatives" does not envisage, for the performance of this activity, any additional professional figure with a support function“.
Already in 2017 the legal offices of AIFA had expressed their opinion in this sense by adding that the "social function of drugs in the protection of the public interest in health, must maintain a high degree of autonomy in carrying out the information service for doctors, in order not to be conditioned by interests  purely private business.
purely private business.
With the'ordinance no. 33428 of 11 November 2022 the Court of Cassation states that it is "the assessment was incorrect in deeming his being subjected to the marketing management and to reasons consistent with the professionalism [of the ISF]. commercial” (point 2 of the considerations of the Court)
Farmindustria itself in document for the certification of scientific information activity he claims: "As a general rule, non-promotional interactions must be carried out in compliance with the applicable law and regulations, guaranteeing the prohibition of carrying out any form of advertising of the drug as defined pursuant to Legislative Decree 219/2006".
The State Council. Section V Sen. 2755 of 04.30.2009 he claims: "in the several times cited Consolidation Act on medicines (legislative decree 219/06), it is inspired by the principle of freedom of scientific information of which the direct relationship between the scientific representative and the doctor constitutes an irrepressible corollary, a relationship that must always take place in full freedom and autonomy and which could therefore not be mediated by third parties" and goes on: "in the exercise of the information activity, the ISF is not accompanied by other subjects, whose presence could evidently discolor the profile of the scientific study on the properties of the drugs, to the benefit of their mere commercial promotion“.
The Council of State once again reaffirms what has already been expressed in the Guidelines for the regional regulation of scientific information on drugs “The ISFs must carry out their work with doctors alone; the presence of the area manager or other professional figures not related to the scientific information activity is allowed only for functions other than scientific information” that is, if a third party is present
one cannot speak of scientific information.
 Even the Novartis has reached an agreement with the RSU which states: "With mutual satisfaction we have reached an agreement which from TODAY ESTABLISHES THAT MEDICAL INFORMATION CAN ONLY BE CARRIED OUT BY ISF AND ALONE, in compliance with the state-region agreements already mentioned in the previous communication and the numerous rulings on the subject and therefore in compliance with the Novartis”.
Even the Novartis has reached an agreement with the RSU which states: "With mutual satisfaction we have reached an agreement which from TODAY ESTABLISHES THAT MEDICAL INFORMATION CAN ONLY BE CARRIED OUT BY ISF AND ALONE, in compliance with the state-region agreements already mentioned in the previous communication and the numerous rulings on the subject and therefore in compliance with the Novartis”.
I would say that on the nature of the tilings Stem there is nothing else to add. If not to proceed through legal channels to enforce the law if a dutiful rethinking is not initiated by Angelini Pharma and all those companies that want to take paths that do not comply with the law and regulations
Related news: STEM. Optimizing and prioritizing field access activities to align with brand direction
STEM. Strengthen your account management based on in-depth insights
Note:
STEM Healthcare is a global company headquartered in the UK and present on six continents. STEM developed an audit process (“independent” evaluation) and built a benchmarking database with over 300,000 completed face-to-face observations in 59 countries. The benchmark provided is unique in the pharmaceutical sector and includes over 140 KPIs (Key Performance Indicators, in Italian key performance indicators, specific metrics that measure the progress of business processes with respect to the set objectives), focusing on 2-3 key priorities when formulating action planning to help clients further accelerate performance. (from the STEM site)
Related news: Angelini Pharma: Erik Lommerde is International Chief Commercial Operations Officer
Epilepsy therapies: Angelini invests 505 million in the Japanese Jcr Pharmaceuticals