The so-called IMS data are useful for the pharmacy, which can thus define its purchasing and sales strategies, but also for companies in the supply chain and their marketing operations. Furthermore, the same data can be used by third parties for statistical or research activities, AIFA itself uses them for its OsMed (observatory on medicines).
IMS Health (now IQVIA) is the leading data provider from which companies purchase consulting services in the form of insights and analytics.
 The salary of the scientific informant/agent is improperly called "commission", the percentage of which is equally improperly established through the IMS data.
The salary of the scientific informant/agent is improperly called "commission", the percentage of which is equally improperly established through the IMS data.
The IMS data are expressed in units (number of packs sold) and value (economic quotation of the sold) but the value data can change on the basis of particular discount campaigns or activities carried out by the pharmaceutical company; this is why often many ISF/agents do not find a correspondence between what is promoted and what is actually invoiced.
In the pharmacy, the products essentially arrive from 2 channels: the pharmaceutical company and the wholesaler. Sell-in data is an estimate of the packs entering the pharmacy through direct sales (pharmacies buy directly from the pharmaceutical company) and indirect sales (pharmacies buy from wholesalers, who in turn buy from companies).
Sell-out data is instead an estimate of the sales of pharmacies to patients e
in fact they represent what comes out of the pharmacy. The difference between sell-in and sell-out is the products not yet sold, in stock at pharmacies.
Ims Health sells monthly flows per microbrick (unit/value) for each pharmaceutical class (market) and for each pharmaceutical specialty (product).
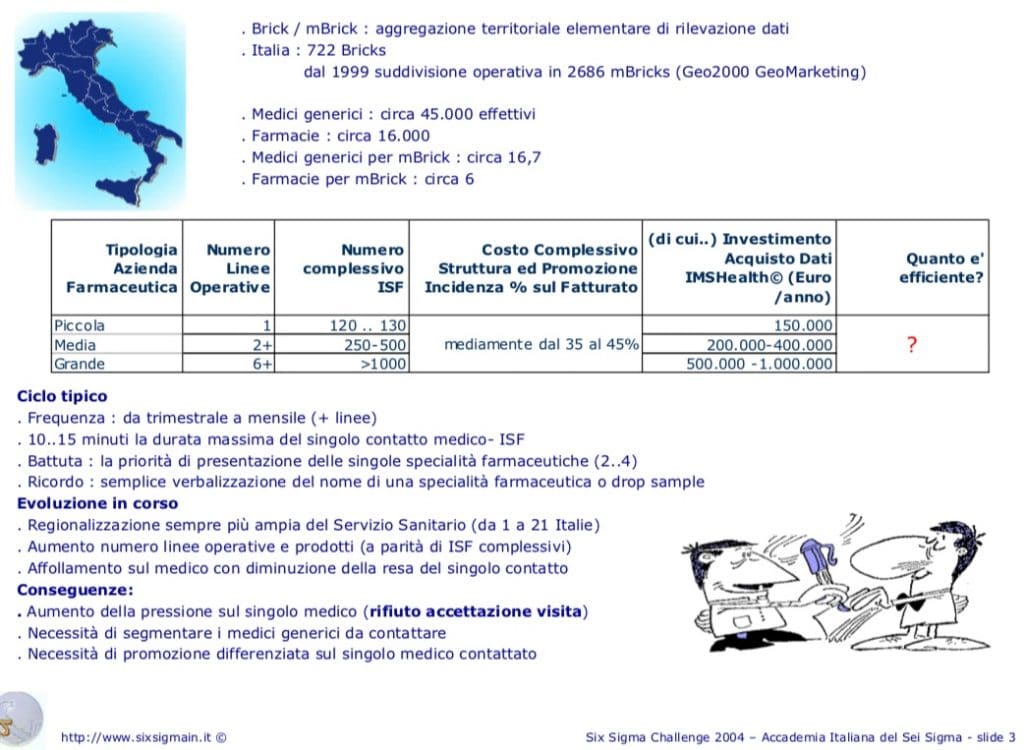
In Italy there were 722 Bricks, since 1999 the operational subdivision is 2686 mBricks. The purchase of IMSHealt data (Euro / year) involves an expense, which for small companies with 120/130 ISF, is € 150,000; medium with 2 or more lines and 250/500 ISF € 200/400,000; large with 6 or more lines and with >1,000 ISF €500,000/1,000,000.
It is not possible to know the sales of drugs in each pharmacy because it could influence the world of sales in an improper way; therefore you can only have minimal territorial aggregations of several pharmacies.
Pharmaceutical companies have their own "customization" of these databases.
Given these characteristics, it is impossible for anyone who is not the buyer of this data to be able to provide it. One way to verify the veracity of the data provided by your company could be to compare them with a colleague who has the same area of expertise and with the same type of drugs.
However, a statement from IMS HEALT confirms that, despite the accuracy of the data collection, the IMS data have only statistical/mathematical value and are not reliable for evaluating an ISF. In fact, IMS HEALTH declares that at a national level the data has a precision difference of approximately 95%, with a margin of error of 5% more or less, at the ASL level the precision decreases, at the Microbrick level it decreases further where it presents a 'wide fluctuation. The only real fact is that of Federfarma or the ASL.
Given the particularity of the type of mandates of the ISF/agents, it is advisable to have an expert lawyer analyze the procedures governing the negotiating relationship between the agent and the pharmaceutical company, in order to carefully evaluate the conduct to be followed in the event of termination of the mandate.
However, it is useful to remember that in Legislative Decree 219/06, in the Regional Regulations, in the CCNL, in AIFA clarifications and in various Court and Cassation rulings, a single profile of the ISF emerges: the mission is to inform, not sell and therefore no ISF can be (officially) licensed for sales, especially on IMS data.
It should also be considered that if an ISF/agent can prove that he is required by the principal to provide his work programme, his itineraries, reports from the doctors visited, etc., he can take legal action to demonstrate that he actually carried out activity employed by the company and the autonomous agent contract was a "false VAT number", with all that follows (wages not received, allowances, refunds, benefits, etc.).
For further information see:
https://www.fedaiisf.it/non-agente-commercio-linformatore-scientifico/;
https://www.fedaiisf.it/wp-content/uploads/2014/12/AIFA.Chiarimenti-su-ISF.pdf