There are 5 vaccines currently authorized and used in Italy in the anti-COVID-19 vaccination campaign:
- Comirnaty (Pfizer/BioNTech), mRNA vaccine authorized since 12/22/2020 and used since 12/27/2020, available in pediatric and adult formulation;
- Spikevax (Moderna), mRNA vaccine authorized since 07/01/2021 and used since 14/01/2021;-
- Vaxzevria (AstraZeneca), recombinant viral vector vaccine authorized since 01/29/2021 and used since 02/01/2021;
- COVID-19 Janssen vaccine (Janssen Cilag), viral vector vaccine authorized since 03/12/2021 and used since 04/22/2021;
- Nuvaxovid (Novavax), inactivated recombinant vaccine authorized since 12/22/2021 and used since 02/28/2022.
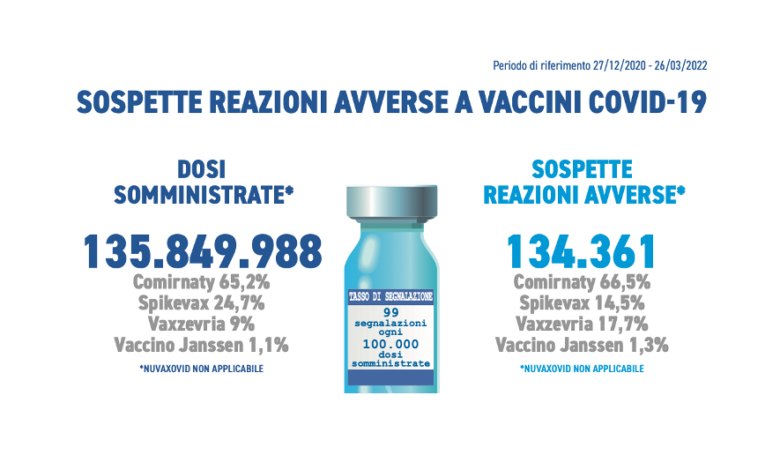
As of 03/26/2022, a total of 134,361 reports of adverse events following vaccination out of a total of 135,849,988 vaccine doses had been entered into the National Pharmacovigilance Network, with a reporting rate of 99 per 100,000 doses administered.
The distribution of reports and doses administered2 by type of vaccine is shown in Table 1.
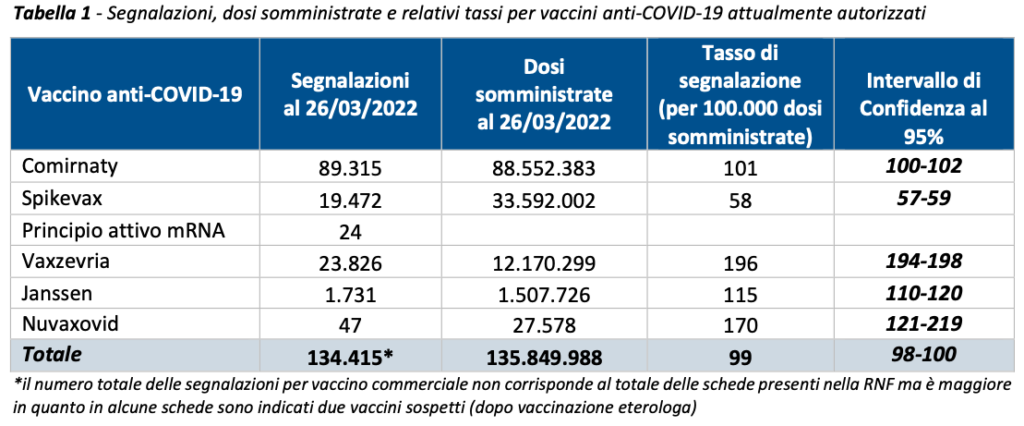
Overall, in Italy Comirnaty was the most used vaccine in this period of the vaccination campaign (65.2%), followed by Spikevax (24.7%), Vaxzevria (9.0%), COVID-19 Janssen vaccine (1.1%) and , recently in use, Nuvaxovid (0.02%). In line with previous Reports, the distribution of reports by type of vaccine follows that of administrations with the exception of Vaxzevria and Spikevax which appear reversed in this trend (Comirnaty 66.5%, Vaxzevria 17.7%, Spikevax 14.5%, COVID-19 vaccine Janssen 1,3%, Nuvaxovid 0,03%).
The trend over time of reports with respect to the number of doses administered (1st-4th dose; with reference to the first quarter of 2022 (Figure 1b), is stable for all vaccines, therefore overlapping with that reported in previous Reports and proportional to the administrations carried out , as evidenced in particular by the similar trend observed for the administrations and reports of suspected adverse reaction to the vaccine booster (3rd dose), albeit on scales with very different orders of magnitude.
Since March, the administration of the 4th dose of vaccine has been underway in some categories of people with frailty (62,214 doses), in relation to which there are only 3 reports, one relating to a therapeutic error in a patient already recently vaccinated with a third dose, one reporting optic neuritis in resolution at the time of reporting and one of vaccine failure in an oncological patient.
Reports in people with previous COVID-19 infection who have received a single dose of vaccine in accordance with ministerial guidelines are assimilated to those referred to the 1st dose of vaccine in the evaluations of this Report.
Please note that the trend illustrated is a snapshot of the reports present in the National Pharmacovigilance Network at the time of data extraction and may change over time.
Report on the surveillance of vaccines against COVID-19 11 27/12/2020 – 26/03/2022





