
In recent days we had published the news of the establishment of the Regulation on Access Methods for Scientific Pharmaceutical Representatives "in the ASP structures of Messina.
We now reproduce the full document: ASP Messina ISF access regulation and the resolution-1855-DG-23-07-2019-1.pdf
We publish an excerpt below
The ISF must be equipped with an identification card.
Companies interested in carrying out medical-scientific information activities in the Sicilian Region must inform Service 7 "Pharmaceuticals" - Department for Strategic Planning of the Health Department, with regard to the professionals indicated above, as required by DDG n.2528/2013.
Dedicated spaces
The activity of the IS/ISF, within the structures of the ASP of Messina, must be ensured and facilitated by the health departments (Departments, Districts, Hospitals), through the identification of suitable premises (doctors' room, department library, doctor's office), in time slots agreed with the Head of the Unit.
 These arrangements must be visibly advertised at the entrance to the facility.
These arrangements must be visibly advertised at the entrance to the facility.
Method of access
For access to the ASP structures of Messina, the IS / ISF and / or operators of the companies producing / distributing DM must show their regional identification card, together with which a personal identification document may be requested validity, under penalty of not being able to be received by the personnel concerned.
The methods of access for reception are communicated through specific notices (Annexes 1, 2, 3), to be positioned near the premises identified for this activity, as well as at the entrance of the individual UU.OO. and in any other room deemed useful to ensure maximum visibility for users as well.
As far as pharmacists working at Direct Distribution points are concerned, reception must take place outside the opening hours to the public, by appointment.
Meeting scheduling
The meetings between the Company's professionals with the IS/ISF and all the other operators of the pharmaceutical companies and the manufacturers/suppliers of medical devices (DM) and in vitro diagnostics (IVD) are carried out by appointment according to the procedures established by the Directors of the UU.OO..
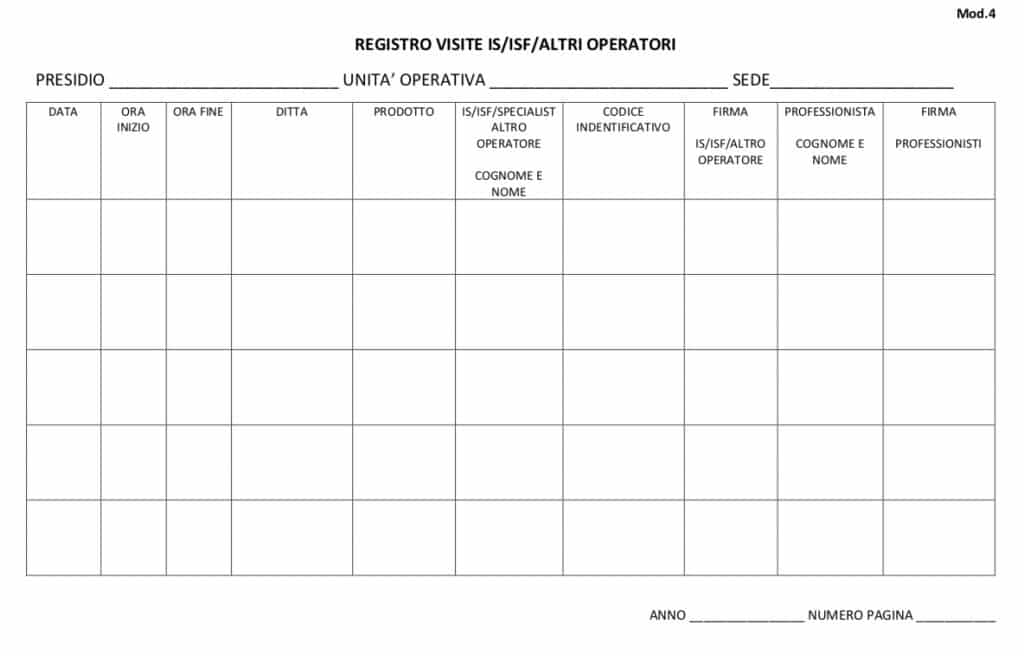 The Director of the Unit, therefore, must prepare an "Attendance Register" (Annexes 4, 5), authenticated by the same, kept at the structure concerned, showing for each line the date, the Company and the name of the IS/ISF/ other operator, the time of entry, the time of exit of the IS/ISF/other operator, the name of the product presented, the name of the receiving doctor.
The Director of the Unit, therefore, must prepare an "Attendance Register" (Annexes 4, 5), authenticated by the same, kept at the structure concerned, showing for each line the date, the Company and the name of the IS/ISF/ other operator, the time of entry, the time of exit of the IS/ISF/other operator, the name of the product presented, the name of the receiving doctor.
The traceability of accesses also allows for the facilitation of on-the-spot checks, aimed at ascertaining compliance with the established time slots.
All IS/ISF and other operators of pharmaceutical companies and manufacturers/suppliers of medical devices (DM) and in vitro diagnostics (IVD) must be informed of the obligation to record accesses in the aforementioned register.
As part of the planning of the meetings, it is hoped that collective meetings organized within the UO/Department concerned will be privileged. The Department and District Heads and possibly the Director of the Pharmaceutical Department and the Clinical Engineer or their delegates can participate in the meetings.
Limits of the activity of the ISFs
The number of individual visits by each ISF/operator of pharmaceutical companies and manufacturers/suppliers of medical devices (DM) and in vitro diagnostics (IVD) to individual medical employees, can be quantified indicatively at a maximum of 5 per year, for each doctor interested in prescribing.
It is possible to derogate from this limit in the event that the Pharmaceutical Company or producer/distributor of DM/IVD needs to communicate to the doctor/professional new and relevant information regarding the appropriate use of its product (new therapeutic indications, adverse events, etc. ...), with particular reference to changes to the Summary of Product Characteristics (SPC), which provide for new therapeutic indications and/or new instructions for use and/or information on safety and on the drug or monitoring device.
IS/ISF cannot carry out any commercial activity at the hospital pharmacy or direct distribution points.
Furthermore, IS/ISF cannot ask the pharmacist for information on the prescribing habits of doctors.
The IS/ISF must carry out their activity individually with the doctors/professionals: the presence of an area manager or other professional figures not related to the activity of scientific information is allowed only for functions other than information (for example, professional tutoring, organization and proposing training events, research projects, etc…).
In order to avoid the dispersion of resources in the case of information activities on individuals, multidisciplinary meetings are preferable, involving several company professionals, in order to deepen certain aspects or to present new aspects.
Companies that are interested in organizing multidisciplinary meetings must submit a request to the Training and Updating Unit and, for information, to the General Management, by means of the appropriate company form (Annex 6), specifying its object and purpose, to be submitted to the competent evaluation of the same can make use of the opinion of the UOC Hospital Pharmaceutical Assistance / Hospital Pharmacy.
In the event that subjects other than company professionals and IS/ISF duly declared according to these regulations must be present at these meetings, participation must be subject to authorization by the Training and Update Unit, after presentation of the identification and professional data relating to these subjects, who justify their interest.
The Department of Medicine ensures the participation of a pharmacist in the multidisciplinary meetings organized in the Company, in order to guarantee the scientific value and the absence of any form, even indirect, of conflict of interest.
Control and monitoring
The supervisory and control activity envisaged by this regulation is guaranteed by:
- the Directors of the UU.OO. involved who guarantee the application and compliance with this regulation, which process the reports received from the professionals involved, in particular those concerning the access requirements of Specialists to operating theaters and similar health areas;
- the Supervision Directorate / Health District / Department which carries out the supervisory and control activity for the part relating to the hygienic-sanitary aspects, which provides support for the management of the activities relating to the reports received regarding the lack of access requirements;
- The Department of Medicine which carries out supervision, control and support activities regarding the activity of scientific information on drugs, aids and devices;
- The Prevention and Protection Service COU and the Qualified Expert Physicist who carry out surveillance and control activities respectively for risk assessment and for aspects relating to ionizing radiation.
- In order to ensure constant monitoring of the activity of the Pharmaceutical Representatives, pharmaceutical companies must notify the Pharmaceutical Department of the ASP of Messina, on an annual basis, by 31 January of the following year:
- the average annual number of visits made by Pharmaceutical Representatives to healthcare operators who were recipients of the Scientific Information activity during the year.
The Department of Medicines, as the structure in charge of control and supervision, draws up a quarterly report on its supervisory activity, highlighting all the critical issues found and sends it to the Company Management.
All violations of this provision and those relating to Legislative Decree no. 541/92 will be communicated not only to the competent authorities, but also to Service 7 "Pharmaceuticals" - DPS Health Department of the Sicilian Region, the Ministry of Health and the Italian Medicines Agency (AIFA), each for the part of its competence.
The OU Directors/Managers are responsible for the correct application of the provisions of these Regulations in their own OU.
The ASP of Messina supervises the correct application of the provisions contained in these Regulations through a multi-professional work group which will carry out internal and external checks, reporting any cases of violation to the Head of Corruption Prevention and Transparency and to the Health Department.
Confidentiality of information
It is not permitted for any healthcare operator employed or affiliated with the SSR to provide the ISFs or other aforementioned operators with information regarding the supplies, consumption of medicines and the prescribing habits and use of the products prescribed or used by doctors concerning the activity under the NHS regime .
Similarly, healthcare professionals are not permitted to provide personal data and/or details of patients to informants, with the exception of what is provided for Specialists on the basis of the instructions provided in the Framework Agreement stipulated pursuant to art. 28 EU Regulation 2016/679.
Anti-corruption legislation and code of conduct
The Companies undertake to respect the Company Code of Conduct, as well as the P.TPCT of the Messina ASP, which can be consulted on the Messina ASP corporate website (www.asp.messina.it) in the "Transparent Administration" section, for the parties competence.
Ed: According to Legislative Decree 196 of the June 30, 2003 (“Privacy Code” – Consolidated Law on Privacy of the Italian Republic), modified from the Legislative Decree 101 of the August 10, 2018 "processing of personal data" means any operation carried out, even without the use of electronic tools, which concerns: the use, consultation, collection,at registration, the organization, storage, processing, modification, extraction, comparison, selection, interconnection, blocking, dissemination, communication, deletion of data, even if not recorded in a database.
The lists of the ISFs, the registration of the visits, the checking times, are personal data of the ISFs. The ISF themselves must give written consent to the keeping of their data, it must also be indicated who holds this data and the person in charge of the same. The security of the data must also be guaranteed, therefore they cannot be exposed to anyone's consultation, least of all to the officials of the companies they work for, this would constitute improper control of an activity on behalf of others. All crimes.
The ISF who are not commercial figures are regulated and limited in their activity, all the commercial figures of the pharmaceutical companies instead have free access. A winning strategy!
Related news: Sicily Circular ISF-19302-of-4-3-2019





