Assosalute has published the 2023 report on the numbers and indices of self-medication drugs. The relationship yes
 structure in 5 chapters. It starts from a European-level examination of the health and economic situation, to then narrow the focus to the Italian pharmaceutical market in 2022 and therefore to the market for products that do not require a prescription. The fourth chapter focuses on the 2023-25 spending trends of the "non-prescription" market and then analyzes the characteristics of the market and the players in the sector.
structure in 5 chapters. It starts from a European-level examination of the health and economic situation, to then narrow the focus to the Italian pharmaceutical market in 2022 and therefore to the market for products that do not require a prescription. The fourth chapter focuses on the 2023-25 spending trends of the "non-prescription" market and then analyzes the characteristics of the market and the players in the sector.
Let's leave out the European market and bring back the Italian market
The Italian market
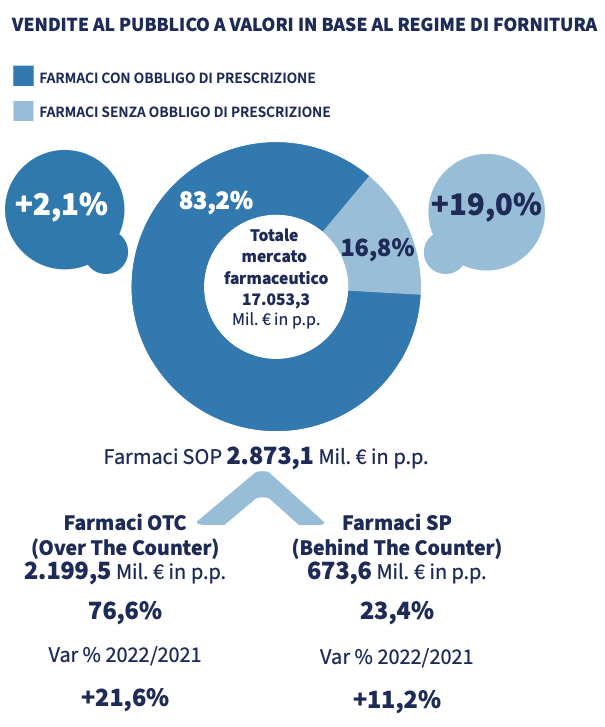
Sales data for 2022 show a dispensing of just under 1.8 billion packs of medicines, for a value of more than 17 billion euros. The data was positive for both prescription and non-prescription drugs. The increase in spending was higher for the latter (+19%) than for the former (+2.1%). "As regards the packs dispensed, the trends are similar to those with values: prescription drugs show a moderately positive trend (+2.0%), while non-prescription medicines show a marked increase, with a double-digit increase (+15.9%)» reads the report.
2022 strengthened the positive trend that had already started in April 2021, thanks to the easing of the restrictive measures imposed by Covid-19, the recovery of the circulation of flu and flu-like viruses. More generally, by the incidence of non-serious disorders. As for the market, the pharmacy has a stable market share of 89% in volumes and 90.5% in values of non-prescription medicines. Although growing, e-commerce is still marginal in this sector.
The innovation of the market "non-prescription”
Compared with the traditional pharmaceutical market, that non-prescription shows more contained margins of innovation, even if the companies in the sector invest constantly in order to innovate and renew the drugs already on the market, to optimize their dosages and forms of administration and, therefore, their effectiveness, reducing any contraindications and interactions. It follows that they are frequently 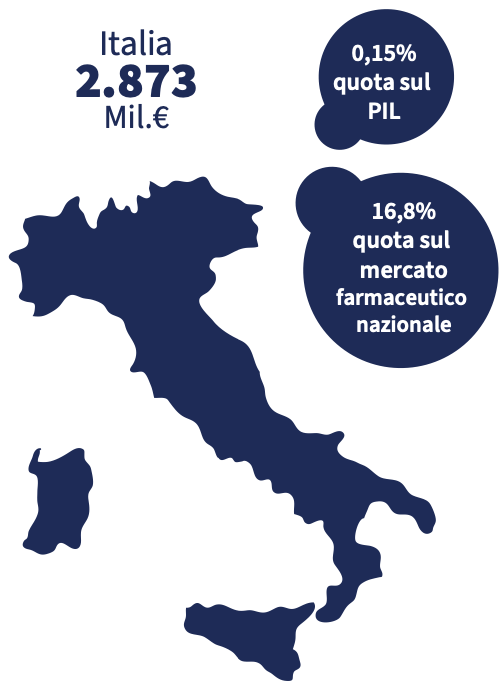 medicines placed on the market whose active ingredient has been on the market for years, but with new pharmaceutical forms or combinations. In terms of turnover, 54.1% is represented by packs launched in 2000 while those launched since 2010 represent 36% of total sales.
medicines placed on the market whose active ingredient has been on the market for years, but with new pharmaceutical forms or combinations. In terms of turnover, 54.1% is represented by packs launched in 2000 while those launched since 2010 represent 36% of total sales.
The 5 most used product categories
In terms of products, the five therapeutic categories with the highest incidence of expenditure and consumption (90.1% of packages sold and 87.2% of expenditure) are those of medicines for the treatment of colds, analgesics, gastrointestinal medicines, dermatologicals and medicines for the circulatory system.
Trends 2023-2025
In view of the given conditions, it is a growth trend is expected for the current year (+9.7%), a positive trend which, albeit at lower rates, will also be maintained in the years to come (+5% and +3.8% respectively in 2024 and 2025). At the 'basket' level, in the absence of changes of a regulatory nature, no changes are expected in the composition of expenditure. Finally, geographically, non-prescription drugs will maintain their current configuration: 50% in the North, 21% in the Center and 27% in the South.
Source Pharmacy News of July 10, 2023
NUMBERS AND INDEXES OF SELF-MEDICATION – ED. 2023
Chapter 1
The health and pharmaceutical picture in Europe in 2022
 Chapter 2
Chapter 2
The pharmaceutical market in Italy in 2022
Chapter 3
The market non-prescription in Italy in 2022
Infographic 1
Chapter 4
Forecasts on the expenditure and consumption of non-prescription drugs: the three-year period 2023-2025
Infographic 1
Chapter 5
Economic structure of the pharmaceutical sector non-prescription and market players
Infographic 1 Infographic 2
Appendix
Regulatory framework
DOWNLOADS
Classification for sale
The reference law, Legislative Decree 24 April 2006 n. 219, commonly referred to as the Drug Code, does not contain criteria on the basis of which a drug can obtain the status of "drug without prescription" (OTC and SOP).
The guidelines issued at European level and Circular no. 13 of the Ministry of Health of 16 October 1997 (on self-medication medicines) allow, however, to identify the following general principles:
- contain active ingredients (and relative dosages) that are well known, widely and consolidated in use, of proven efficacy and whose safety profiles are well known;
- they are intended for the treatment of simple symptoms/disorders, recognizable from common experience, not serious and which tend to be solvable in a short time.
The combined provision of the provisions of Legislative Decree 219 has led to a dual classification of non-prescription drugs”:
- Self-medication or OTC those that can communicate to the public (advertising) and are freely accessible at the point of sale (self-service);
- SOP those that can communicate to the public (advertising) but are not freely accessible at the point of sale (self-service)*.
The Authority responsible for the authorisation/registration of a non-prescription drug is the Italian Medicines Agency (AIFA), which also establishes its classification as a self-medication/OTC or SOP drug.
 With the Law of 16 November 2001 n. 405, an "identification sticker" was introduced, which must be shown on the packaging of all non-prescription drugs (self-medication/OTC and SOP).
With the Law of 16 November 2001 n. 405, an "identification sticker" was introduced, which must be shown on the packaging of all non-prescription drugs (self-medication/OTC and SOP).
The adoption of the sticker took place, as required by Law 405/2001, with a Decree of the Ministry of Health (1 February 2002) which also defines the graphic characteristics and dimensions of the sticker to be inserted, visibly, on the packages .
1 As medicines, even self-medication ones must comply with all the national and European rules that regulate the pharmaceutical sector: efficacy, safety of use for the use for which they are intended, quality and production standards.
2 Legislative Decree 24 April 2006 n. 219 of "Implementation of directive 2001/83/EC (and subsequent amendments) relating to a Community code concerning medicinal products for human use, as well as of directive 2003/94/EC".
3 In this text, the acronym AM is also used for OTC drugs. Non-prescription non-self-medication drugs are indicated with the acronym SP while the notation SOP is used to indicate the set of all Non-Prescription Drugs (see Legend).





