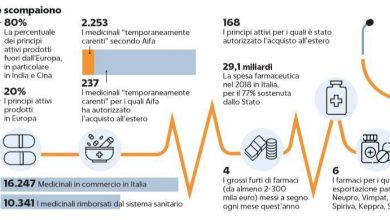
Nas checks are back in warehouses and deposits of pharmaceutical distributors to verify compliance with legal requirements. The campaign, conducted in agreement with AIFA, should have a wider range of action than the previous one, which was concentrated above all in central Italy; the new operation, on the other hand, aims to cover a greater number of regions, even if there is an understandable reservation about its real extent. In any case, the objective remains the same, and that is to bring out those companies that do not comply with the structural requirements dictated by Legislative Decree 219/2006 and therefore, probably, use the wholesaler authorization only to carry out parallel trades. The campaign conducted by the Nas last year had brought out some striking cases, such as the distributor who had only three or four brands of drugs in stock, the most requested by exporters, or the company whose warehouse was reduced to a simple pallet , housed in a shed shared with several wholesalers.
Nas checks are back in warehouses and deposits of pharmaceutical distributors to verify compliance with legal requirements. The campaign, conducted in agreement with AIFA, should have a wider range of action than the previous one, which was concentrated above all in central Italy; the new operation, on the other hand, aims to cover a greater number of regions, even if there is an understandable reservation about its real extent. In any case, the objective remains the same, and that is to bring out those companies that do not comply with the structural requirements dictated by Legislative Decree 219/2006 and therefore, probably, use the wholesaler authorization only to carry out parallel trades. The campaign conducted by the Nas last year had brought out some striking cases, such as the distributor who had only three or four brands of drugs in stock, the most requested by exporters, or the company whose warehouse was reduced to a simple pallet , housed in a shed shared with several wholesalers.
But in addition to the Nas controls, Aifa is preparing to implement other tools as well to counter the fake distributors. This is the case of the Memorandum of Understanding between the Medicines Agency, Regions and acronyms of the supply chain (manufacturers, distributors and pharmacies) which summarizes the regulatory framework within which operators and administrations  they have to operate. In summary, the draft agreement reiterates the current regulations in points and from these it deduces a series of principles on which the Regions and the supply chain agree to meet: the companies authorized for wholesale distribution must hold at least the 90% of the drugs on the market, including of table 2 (which require a separate authorization from the Ministry of Health); Wholesaling and sale to the public must be kept separate and the premises of the distributor must be different from those of the pharmacy. Similarly, two distributors with separate authorizations cannot share inventory, minimum equipment and personnel.
they have to operate. In summary, the draft agreement reiterates the current regulations in points and from these it deduces a series of principles on which the Regions and the supply chain agree to meet: the companies authorized for wholesale distribution must hold at least the 90% of the drugs on the market, including of table 2 (which require a separate authorization from the Ministry of Health); Wholesaling and sale to the public must be kept separate and the premises of the distributor must be different from those of the pharmacy. Similarly, two distributors with separate authorizations cannot share inventory, minimum equipment and personnel.
The objective of the protocol, now close to being signed, is twofold: on the one hand, to facilitate and direct the authorization activity of the Regions, so that "oversights" of the past are not repeated (wholesale authorization requests presented by different subjects who report the same address for the warehouse will be subjected to careful verification) , on the other hand, to offer the administrative courts (Tar and Council of State) a reference in the event of appeals by distributors who have had their authorization revoked (in Lazio and Sicily, some appeals have been suspended).