Despite the uncertainty of the regulatory framework, some companies are starting to use social media. Here are the main rules to take into consideration when developing digital strategies
by Luca Tosoni, lawyer, Covington & Burling LLP – 20/03/2017 – Digital Agenda
With the development of the Internet and social networks, the communication channels available to the pharmaceutical industry have widened. However, until now, pharmaceutical companies have been particularly cautious about using social networks. According to one study of the IMS Institute for Healthcare Informatics, in 2014, only half of the top 50 pharmaceutical companies were active on social media. The distrust of companies is combined with that of the medical profession. In fact, a recent one investigation conducted by the Study Center of the Italian Federation of Family Doctors (FIMMG) revealed how the majority of the doctors interviewed had a negative opinion of scientific information via social media.
 The reluctance of pharmaceutical companies to use these new communication channels can in part be explained by the absence of clear regulation on the subject, with the relative risks that this entails. The Ministry of Health has recently updated its own guidelines to the use of new means of diffusion in healthcare advertising precisely to address the theme of social networks, but some issues still remain to be clarified.
The reluctance of pharmaceutical companies to use these new communication channels can in part be explained by the absence of clear regulation on the subject, with the relative risks that this entails. The Ministry of Health has recently updated its own guidelines to the use of new means of diffusion in healthcare advertising precisely to address the theme of social networks, but some issues still remain to be clarified.
Despite the uncertainty of the regulatory framework, some companies are starting to direct their communication strategies towards a greater use of social media. While waiting for a clarifying intervention by the legislator, it therefore seems appropriate to review what are today the main rules to be taken into consideration in the development of digital strategies in the pharmaceutical field.
Advertisement of medicines on social media: the advertising of medicinal products is regulated by Legislative Decree 219/2006. The Legislative Decree defines medicinal product advertising as “any information, customer research or exhortation action, intended to promote the prescription, supply, sale or consumption of medicinal products” both among the public and among the medical profession. This definition is not limited only to some means of dissemination and therefore can also be applied to promotional messages on social media. It follows that the advertising rules established by Title VIII of Legislative Decree 219/2006 also apply to promotional activities on social networks. For example, given the opening of social networks to the general public, only those drugs not subject to medical prescription can be promoted on social networks, provided that the manufacturing company or company responsible for placing them on the market has obtained a specific authorization from the of the Ministry of Health.
The guidelines of the Ministry mentioned above further limit the use of social networks to promote pharmaceutical products. In fact, they admit it only in the following two cases.
The first concerns Facebook. On this social network, authorized promotional messages can only be disseminated in the right column of the "wall" of the desktop version of Facebook. In reverse, Businesses cannot post about their products on their Facebook business pages.
The second concerns Youtube, where advertising messages can be disseminated provided that they have obtained prior authorization from the Ministry of Health and the interactivity features are deactivated (like, share, comment).
These limits apply only to individual product advertising and not to corporate advertising or disease awareness messages.
 Interaction with the public through social media: the rules on advertising referred to in Legislative Decree 219/2006 do not apply to "correspondence necessary to respond to a precise and unsolicited request for information on a specific medicinal product." Therefore, companies can legitimately respond to requests for information about their products that they receive from doctors and patients, provided that such responses are contained and have no promotional characteristics. However, the term "correspondence", if reduced to the reality of social networks, suggests that answers to questions about pharmaceutical products are allowed only through private chats and not instead on the open pages of social networks (for example in the comments on the Facebook page of a pharmaceutical company). In any case, care must be taken that requests for information are not solicited.
Interaction with the public through social media: the rules on advertising referred to in Legislative Decree 219/2006 do not apply to "correspondence necessary to respond to a precise and unsolicited request for information on a specific medicinal product." Therefore, companies can legitimately respond to requests for information about their products that they receive from doctors and patients, provided that such responses are contained and have no promotional characteristics. However, the term "correspondence", if reduced to the reality of social networks, suggests that answers to questions about pharmaceutical products are allowed only through private chats and not instead on the open pages of social networks (for example in the comments on the Facebook page of a pharmaceutical company). In any case, care must be taken that requests for information are not solicited.
Scientific information 2.0: the guidelines for the use of new means of dissemination in healthcare advertising clearly establish that information on the drug addressed to healthcare professionals must be accessible only to the aforementioned operators, even when it is disseminated via the Internet. This does not go well with the free access to the general public which is typical of most social networks. However, a marginal use of social media for scientific information purposes remains possible. For example, by creating closed groups on Facebook or LinkedIn which can only be accessed by those operators who have sent the data necessary for their identification. Obviously, the promotional material distributed within these groups must first be filed with AIFA.
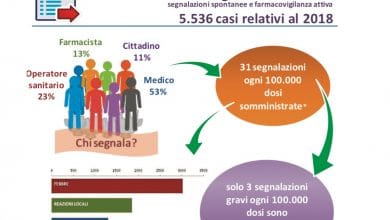
Pharmacovigilance: according to recent studies, almost one in three patients discuss their health problems on the Internet and Facebook is becoming one of the main sources of health information. In consideration of this phenomenon, the Guideline on Good Pharmacovigilance Practices (GVP) Module VI of the European Medicines Agency has introduced the obligation for pharmaceutical companies to carry out regular screening of social media under their control or responsibility at the Search for potential adverse reaction reports to be notified to Regulatory Authorities.
Privacy and profiling: another aspect to which it is important to pay particular attention is that of privacy. The subject of the processing of personal data is well known to operators in the sector. Here it is sufficient to recall that according to the Privacy Code in force, the processing by private individuals of data relating to health, even if made public, normally requires the informed consent of the interested parties. Therefore, a pharmaceutical company will be able to use the health data that users spontaneously make public on the company's Facebook page, for purposes other than those imposed by legal obligations, only with the informed consent of the users in question. Article 9 of the General Data Protection Regulation (GDPR) mitigates this requirement by allowing the processing of sensitive data without consent if the processing concerns “personal data manifestly made public by the data subject.” However, this treatment must remain in compliance with the principles of lawfulness, purpose, relevance and non-excess.
 Another issue related to privacy is that of profiling. The Privacy Code does not define it, but imposes an obligation to notify the Guarantor to all data controllers who process data with the aid of electronic tools for profiling purposes. In addition, the Guarantor has got to clarify that in order to process personal data relating to the prescriptive attitudes of doctors, for profiling and promotional purposes, it is necessary to provide suitable information to the interested parties, and to obtain valid consent from them. The same principles should apply to information about their prescribing attitudes that doctors share on social networks. The GDPR also contains specific rules on profiling. However, most of these rules only apply to profiling that produces legal effects or significantly affects the person concerned.
Another issue related to privacy is that of profiling. The Privacy Code does not define it, but imposes an obligation to notify the Guarantor to all data controllers who process data with the aid of electronic tools for profiling purposes. In addition, the Guarantor has got to clarify that in order to process personal data relating to the prescriptive attitudes of doctors, for profiling and promotional purposes, it is necessary to provide suitable information to the interested parties, and to obtain valid consent from them. The same principles should apply to information about their prescribing attitudes that doctors share on social networks. The GDPR also contains specific rules on profiling. However, most of these rules only apply to profiling that produces legal effects or significantly affects the person concerned.
Patient enrollment in clinical trials: social media could also become an important tool for enrolling patients in clinical trials of medicines. This is what some scholars claim item recently published in JAMA Oncology. However, in this regulatory framework, "the procedures for enrolling subjects and the information procedures for disseminating knowledge of the trial" must be approved by the competent ethics committee. Therefore, it remains to be seen what position the Italian ethics committees will take if they are offered enrollment on Twitter or Facebook.
Final considerations: the traditional legislation on the advertising of medicines was not written with social media in mind and it should not be overlooked that drawing the line between information and advertising is even more difficult in this context. Furthermore, in some circumstances, the principle of freedom of expression, referred to in Article 11 of the Charter of Fundamental Rights of the European Union, could impose a less rigid interpretation of the aforementioned legislation given that the same also applies to the dissemination by of an entrepreneur of information of a commercial nature, in particular in the form of advertising messages.
Finally, it should be emphasized that discussions on social networks can also have an impact in determining which uses of a drug fall within those to be considered reasonably foreseeable for the purposes of legislation on liability for defective products.
Related news: Internet advertising on OTC, the Ministry updates guidelines
 The emotional sale in the scientific information of the drug
The emotional sale in the scientific information of the drug