Research prospects, remuneration of innovation and access to therapies
Rome, 20 April 2015, 10.30-13.00
Censis – Piazza di Novella 2 – Rome
Introduces:
Joseph DeRita– Censis President
 Presents research:
Presents research:
Carla Collicelli – Secretary General Forum for Biomedical Research
They discuss:
Massimo Scaccabarozzi – President Farmindustria
Pierluigi Navarre – Catholic University of Sacred Heart
Stefano Vella – Director of the Department of Medicines, Iss
Americo Cicchetti – Catholic University of Sacred Heart
Francesco DeLorenzo – President Favo
The prolongation of life and the high expectations of citizens, patients and health professionals urgently raise the issue of enhancing therapeutic and pharmacological innovation and that of the effective availability of new drugs for patients who need them. The study by the Forum for Biomedical Research of Censis deals with the most important aspects of the issue: role of the subjects involved in the various phases of the process, priority work paths for basic and applied research, evolution of regulatory processes, methods of remuneration for innovation in the market introduction phase, patient access to innovative therapies. Original data will be illustrated on innovative molecules and on patients' problems in accessing medicines.
Healthcare: hundreds of innovative drugs are being studied to treat many diseases, but authorization procedures and spending reviews are slowing down their spread
According to the 35% of the Italians, public coverage for medicines is insufficient, according to the 79% there are too many medicines for serious pathologies at the expense of the patients. And when a new active ingredient is discovered, on average after 15 years of research, it takes an average of 427 days after approval at the Community level to get to effective use (only 109 in the UK)
R Rome, 20 April 2015 – In the last thirty years, life expectancy has increased by 6.5 years for women and 8 years for men, reaching an average of 85 and 80 years respectively. Over time, survival from many pathologies, both acute and chronic, has improved significantly. And the demand for increasingly effective treatments continues to grow. The diseases most feared by Italians today are tumors (62.6%), those that cause non-self-sufficiency (30.7%), cardiovascular diseases (28.3%), neurological diseases and dementias (26.3%). Italians have high expectations of medicines, which according to their opinions must mainly cure diseases (36.7% thinks so), help improve the quality of life (20.9%), help to live acceptably with pathologies (19,5%). This is what emerges from the 2015 Biomedical Monitor, the survey conducted periodically by Censis as part of the Forum for Biomedical Research which takes stock of the key issues of Italian healthcare.
Rome, 20 April 2015 – In the last thirty years, life expectancy has increased by 6.5 years for women and 8 years for men, reaching an average of 85 and 80 years respectively. Over time, survival from many pathologies, both acute and chronic, has improved significantly. And the demand for increasingly effective treatments continues to grow. The diseases most feared by Italians today are tumors (62.6%), those that cause non-self-sufficiency (30.7%), cardiovascular diseases (28.3%), neurological diseases and dementias (26.3%). Italians have high expectations of medicines, which according to their opinions must mainly cure diseases (36.7% thinks so), help improve the quality of life (20.9%), help to live acceptably with pathologies (19,5%). This is what emerges from the 2015 Biomedical Monitor, the survey conducted periodically by Censis as part of the Forum for Biomedical Research which takes stock of the key issues of Italian healthcare.
On the other hand, the perception of Italians regarding the public coverage of medicines is problematic. The availability of medicines guaranteed by the National Health Service is judged insufficient by 35.2% of citizens (and the percentage rises to 53.8% among less educated people). The 78,8% believes that there are too many drugs needed for serious pathologies affecting patients. 83% thinks the ticket penalizes sick people. The 58% claims to have experienced an increase in out-of-pocket healthcare spending in recent years. And 65% indicates drugs as an item of expenditure on the rise for households. Today the share of Italians who have reduced the purchase of drugs to be paid for out of pocket is equal to 27.6%.
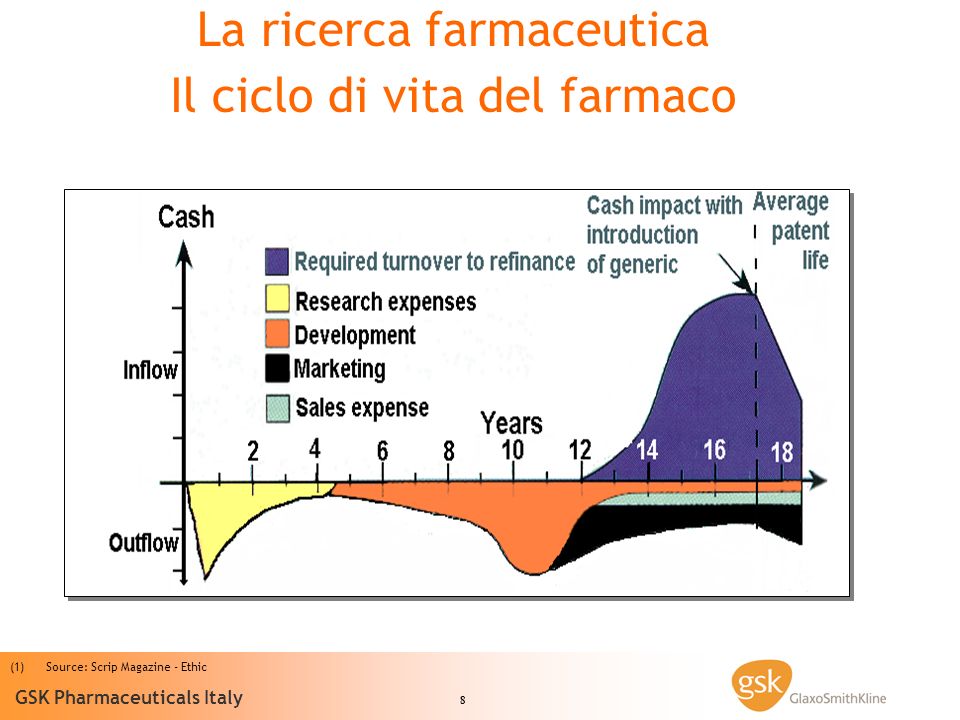 About 15 years of research are needed to make a new drug available. Only one new molecule out of every 10,000 tested successfully passes the many tests required to be approved as a medicine. But then, at the end of this process, in Italy it takes too long to access the new medicines, after they have been approved at the community level: 427 days on average, against 364 in France, 330 in Spain, 109 in the United Kingdom.
About 15 years of research are needed to make a new drug available. Only one new molecule out of every 10,000 tested successfully passes the many tests required to be approved as a medicine. But then, at the end of this process, in Italy it takes too long to access the new medicines, after they have been approved at the community level: 427 days on average, against 364 in France, 330 in Spain, 109 in the United Kingdom.
Research in the oncology field is the most widespread in the world: it is expected that by 2018, studies in the clinical trial phase for 38% will be concentrated on oncology, while all other sectors will remain below the 10% threshold. Experimentation on neoplastic pathologies is also the main research area in Italy. In our country in 2012, 697 clinical trials were underway for the testing of innovative drugs, financed for 67.7% by companies and for 32.3% by non-profit organizations. In 2013, the number of ongoing clinical trials was 583, with a prevalent concentration in the area of neoplasms (35%). 403 biotechnological products are also being studied, of which 169 in the oncological area.
The investments in research and development promoted by the pharmaceutical industry in Italy amount to 1.2 billion euros, equal to 4.2% of the total investments made in Europe, while the number of employees employed in these activities is equal to 5,950 (5 ,5% of the total). More resources are invested in the main European countries (19.1% of investments in European research and development in Germany, 18.1% in the United Kingdom, 15.3% in France) and a higher number of workers are employed (21.2% in the UK, 18.8% in Germany, 18.7% in France).
The problem of the sustainability of the national health service's circus costs for innovative drugs remains unresolved. These are high costs, especially when the number of recipient patients is large. Direct investments can exceed one billion euros, reaching 2.6 billion if the cost of capital invested in research is added. But only 2 out of 10 innovative drugs allow R&D costs to be amortised. The recent case of the anti-hepatitis C drug (Sofosbuvir) is emblematic. The cost of a therapeutic cycle is equal to 37,000 euros for public facilities, but the additional government allocation for this therapy has so far been around one billion euros for two years, which is believed to involve around 50,000 patients, compared to an overall audience estimated at around 1.5 million people has contracted the virus and a number of patients diagnosed with hepatitis C in excess of 300,000.
These are the main results of the 2015 Biomedical Monitor, created by Censis as part of the activities of the Forum for Biomedical Research, presented today in Rome by Carla Collicelli, Secretary General of the Forum for Biomedical Research, and discussed by Massimo Scaccabarozzi, President of Farmindustria, Pierluigi Navarra, Director of the Institute of Pharmacology of the Catholic University of the Sacred Heart, Stefano Vella, Director of the Department of Medicines of the Istituto Superiore di Sanità, Americo Cicchetti, Director of Altems of the Catholic University of the Sacred Heart, Francesco De Lorenzo, President of Favo, and Giuseppe De Rita, President of Censis.
Related news:
| Genetic registries and tests: the topics addressed by the rare disease experts of the European Commission |
census. Healthcare: Italians increasingly informed, but resigned to "do it yourself"