The "AIFA in the COVID-19 emergency" report traces the main activities carried out during the pandemic, by  the exceptional ones due to the new and unprecedented tasks which the Agency had to face in the emergency, to the equally significant ones which fall within the typical functions of AIFA and which make it unique on the international scene: from the authorization of new drugs to the governance of pharmaceutical expense; from monitoring use in clinical practice to monitoring the efficacy, quality and safety of products on the market; from the commitment to guarantee essential medicines and innovative therapies to combating medicine shortages; from the consolidation of the international role to the promotion of information and independent clinical research.
the exceptional ones due to the new and unprecedented tasks which the Agency had to face in the emergency, to the equally significant ones which fall within the typical functions of AIFA and which make it unique on the international scene: from the authorization of new drugs to the governance of pharmaceutical expense; from monitoring use in clinical practice to monitoring the efficacy, quality and safety of products on the market; from the commitment to guarantee essential medicines and innovative therapies to combating medicine shortages; from the consolidation of the international role to the promotion of information and independent clinical research.
During the pandemic, the Agency, with the support of its Technical-Scientific Commission (CTS), was called upon to evaluate all clinical trials on medicines for COVID-19 and facilitated, regulated and supervised access to therapies potentially useful. Managed the sudden shortage of some medicines in hospitals and in the community. She has been actively involved in Italy's participation in the joint procurement programs for medicines and vaccines initiated by the EU Commission on behalf of the interested states. It rigorously and promptly authorized drugs and vaccines and monitored their use and their effects in clinical practice.
An extraordinary historical period that the Report tries to tell by condensing the multiple activities of its offices into numbers.
"Speaking of the extraordinary nature of the procedures - underlined, among other things, the Director General Nicola Magrini in the introduction to the volume - it is for me a reason for gratitude and at the same time pride to claim here the commitment made by all the Agency's structures in guaranteeing the faster access to effective and safe anti-COVID-19 medicines and vaccines, in constant contact with the Ministry and the other components of the National Health Service, with the use of completely new operating methods, such as, in an exemplary manner, the continuous meetings of the Commission Technical-Scientific of AIFA, called to evaluate all the protocols of the clinical studies developed during the pandemic ".
AIFA – Published on: 18 November 2022
Report "AIFA in the COVID-19 emergency"
The fight against fake news: the information section of the portal, press releases and FAQs on vaccines
Since the beginning of the pandemic, the Agency has faced the infodemic fueled by uncertainty about the therapies to be adopted in the treatment of the COVID-19 disease. AIFA intervened constantly, with interviews, participation in TV programs, communications and updates on the institutional portal, quickly creating a section of the site dedicated to the health emergency, which soon became a point of reference for clinicians, researchers, information professionals and interested citizens to qualified, validated and updated information. The launch of the portal in English, with the translation of the main published contents, has allowed for a wider sharing of data and information at an international level.
There have been several cases that required the intervention of AIFA to restore scientific foundation to the news circulating in the mainstream media and on the Internet: the alleged interferences between commonly used drugs used for acute or chronic diseases (anti-inflammatories such as ibuprofen and antihypertensives such as sartans) and the evolution of the COVID-19 infection; the presumed effect of antihypertensive drugs (ACE inhibitors and sartans) on the transmission and evolution of the disease; the first information on the efficacy of some drugs used outside the therapeutic indications (tocilizumab, hydroxychloroquine, hyperimmune plasma).
With the launch of clinical trials and the approval of the first vaccines and drugs, all available information was published on the portal and thematic FAQs on vaccines were created, subsequently expanded and updated in the light of new evidence and the decisions taken by the national health authorities.
www.aifa.gov.it/domande-e-risposte-su-vaccini-covid-19
AIFA and scientific information
Independent information for a new drug culture

The Agency's effort was aimed at providing constant, transparent and accessible information on the issues l
linked to drugs and vaccines for COVID-19, having the renewed institutional portal as a pivot and enhancing the role and institutional presence of AIFA on traditional media and social channels.
Other information and communication activities were carried out on specific topics, in particular on the use of antibiotics and on antibiotic resistance.
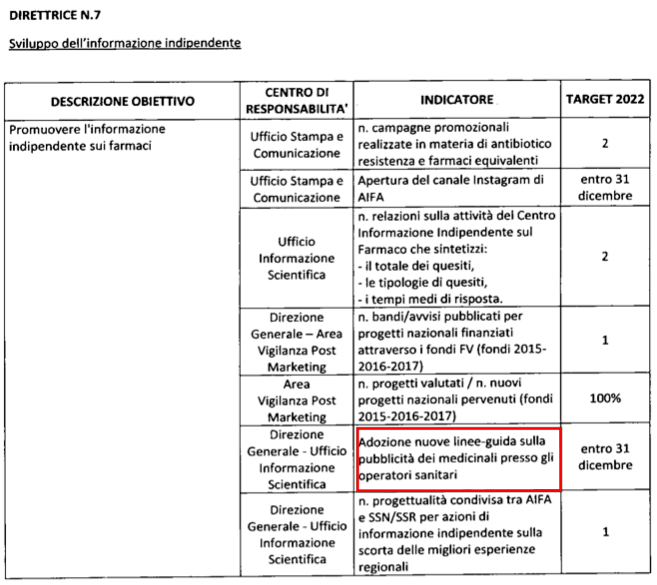 The need to promote independent information on drugs also through targeted projects, disseminated throughout the territory and coordinated by the Agency, has led to the launch of a preparatory activity for the launch of a specific AIFA tender for the financing of a project of national, published in May 2022 and under evaluation at the time of closure of this Report.
The need to promote independent information on drugs also through targeted projects, disseminated throughout the territory and coordinated by the Agency, has led to the launch of a preparatory activity for the launch of a specific AIFA tender for the financing of a project of national, published in May 2022 and under evaluation at the time of closure of this Report.
Indeed, the Board of Directors of the Agency has authorized the use of 5 million euros as part of the funds envisaged by the State-Regions Agreement of 6 June 2019 (for the years 2015, 2016 and 2017), which defines the guidelines for the implementation of an active pharmacovigilance program through the signing of agreements between AIFA and the individual Regions.
The objective of the call is precisely to encourage the creation of a network between central and regional institutions and operators in the scientific information sector that allows the creation of shared awareness and information projects on the efficacy, safety and appropriateness of use of drugs and vaccines. The areas identified by the Agency as priorities are paediatrics, oncology, chronic diseases and antibiotic resistance.
The AIFA portal, information reference point in the COVID-19 emergency
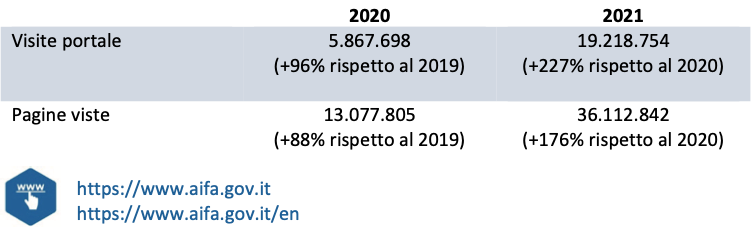
The AIFA portal has been a point of reference for information on anti-COVID-19 drugs and vaccines, with constant updates of contents, reports and FAQs and with the publication of data in an open and interactive format. In 2021, visits to the portal exceeded 19 million, with over 36 million page views, demonstrating the great importance that the contents published on drugs and vaccines for COVID-19 have had in the context of the pandemic emergency. To respond to the information needs of an increasingly large audience of users, with complete and accessible information, the Agency also inaugurated the English-language version of the institutional portal in 2020, thanks to which it offers an additional tool to stakeholders and users international students wishing to keep up to date on AIFA activities. In the two-year period 2020-21, there were over 6,200 publications (and updates) of content and for all the most relevant, the Agency has also made the English version available.
During 2021, the Agency also developed new portal functions for greater transparency and ease of access to information. The renewed site offers content in a more coherent and effective way and with the responsive technology consultation through mobile devices has been improved.
An entire area of the site is dedicated to "AIFA Data", accessible free of charge and available with a distribution license, which allows third parties to distribute, modify and use the data citing the source.
The AIFA site is designed and built in full compliance with the technical requirements and regulatory provisions aimed at facilitating access for subjects using assistive technologies.
Initiatives to promote the correct use of medicines: the OPERA programme
"Fewer antibiotics, more effective" is the theme of the social campaign on antibiotic resistance conducted by AIFA in 2020 to promote the conscious use of these medicines in the general population. Information materials with useful advice and messages were shared by the Agency on social channels, through spots on YouTube, FAQs and postcards on Facebook, Twitter and LinkedIn which spread the key message of the campaign: using fewer antibiotics today means having more effective antibiotics Tomorrow.
On antibiotic resistance, the Agency has activated a working group (called AIFA- OPERA, Optimization of Antibiotic Prescription) which makes use of some of the leading national experts on antibiotics and resistance and which has the task of supporting AIFA in promote optimal and specific uses of antibiotics to preserve their efficacy and reduce the onset of resistance. A very urgent topic during the SARS-CoV-2 pandemic.
The working group's program of activities is divided into different initiatives with achievable objectives in the short (6-12 months) and long-term (next 3-5 years).
Short-term goals are to develop recommendations based on the best scientific evidence of antibiotic therapy of infections, both for hospitals and general practitioners, and to strengthen antibiotic monitoring systems.
Long-term goals are the development of a series of coherent actions, from awareness campaigns to the organization of a network of centers of excellence to support AIFA-OPERA activities in terms of training and research.
Aware of the important challenge that these interventions represent, the Agency intends to provide the maximum scientific support to prescribers, health professionals, citizens and political decision-makers, to spread the culture of the optimal and responsible use of antibiotics.
(p. 23 and following of the Report)
Related news: AIFA-OPERA activation
Note: OPERA stands for Optimization of antibiotic prescription