On 26 September, in Rome, the AboutPharma seminar on the new online pharmacovigilance obligations. The legal obligations of the ISF
Monday 1st September 2014 – HPS
Of Editorial board
Patients are turning to “Doctor Web” more and more frequently; not only to receive diagnoses - often unlikely - or suggestions on remedies and therapies, but also to report side effects or adverse events resulting from the use of medicines. Not infrequently they disperse these reports between Forums and Social Networks: boundless digital spaces, over which it seems difficult to exercise effective and timely surveillance. Difficult, but perhaps not impossible, using the most modern technologies for monitoring the network. The regulations, then, could be updated as a result of technological evolution, since, as the fathers of law taught, no one can be forced to obtain impossible results; but when the boundaries of the technically possible move - and today the Net can actually be monitored with surprising effectiveness - the time has perhaps come to modify the contours of legal obligations accordingly; all the more so when the interest to be protected is patient safety.
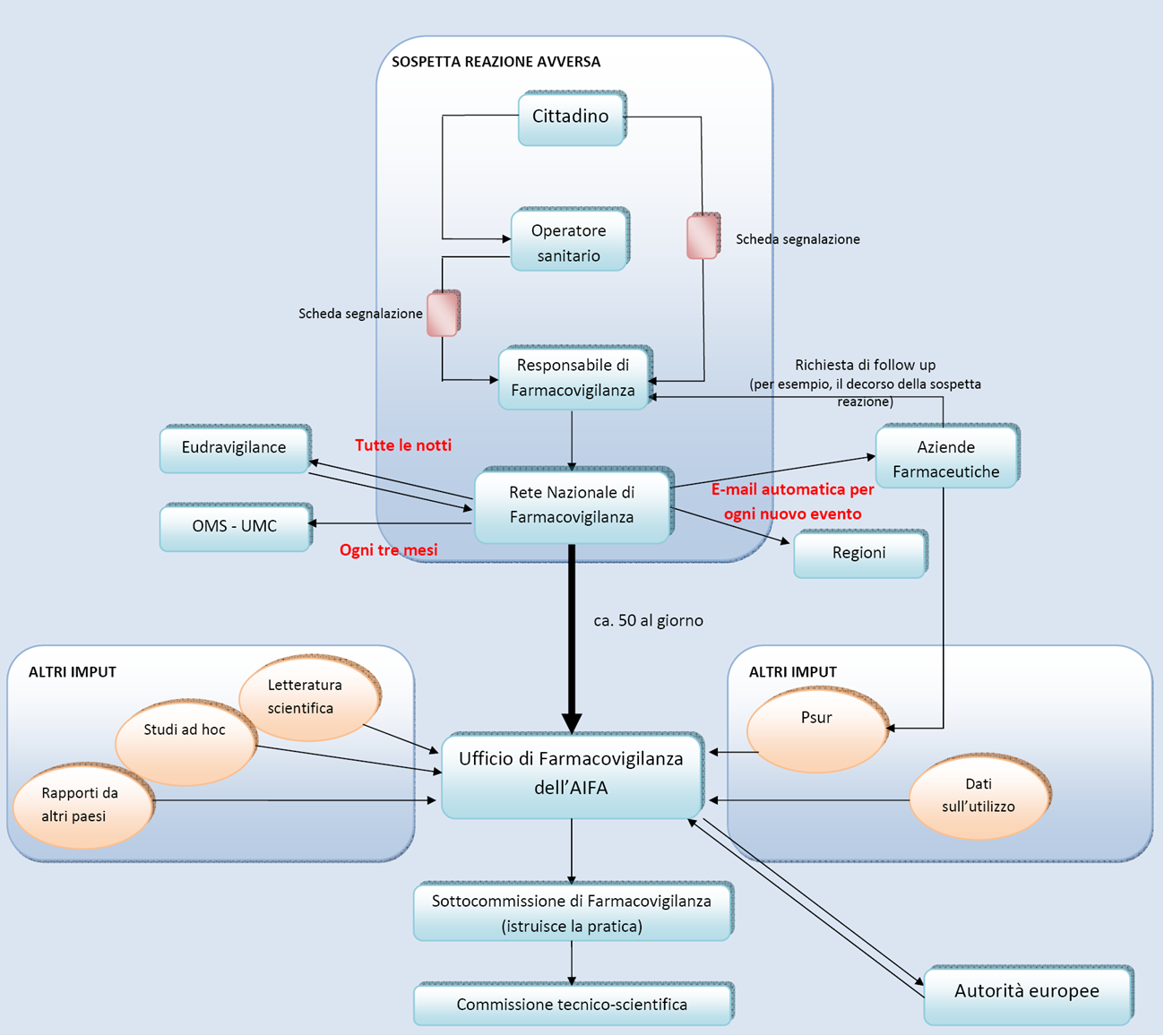
The European Authorities also seem to have moved in this direction, and on the basis of this logic: the EU regulation 1235/2010, the Directive 2010/84/EU and all related acts introduce very important changes to the regulatory system on pharmacovigilance, with the aim of increasing the effectiveness, speed and transparency of interventions. In this context also fits the paragraph VI B.1.1.4. from the guidelines on GVP (Good Pharmacovilance Practices) issued by the EMA (European Medicines Agency). The paragraph is explicitly entitled "Information on suspected adverse reactions from the Internet or digital media" and establishes the obligation for MA holders to regularly monitor the websites and digital media that are online under their own management or responsibility: this, of course, in order to promptly intercept, and turn over to the appropriate authorities, any reports of suspected adverse reactions "disseminated" on the net by patients. On the verge AIFA also intervened, with a press release dated February 7, 2014, recalling the provision of the EMA and affirming the obligation for pharmaceutical companies to consider any information acquired on the Internet as spontaneous reporting of adverse reactions, with the further, consequent obligation to notify the regulatory authorities of those considered valid and reliable.
The AboutPharma seminar, scheduled for next 26 September in Rome, aims to define the precise contours of the new pharmacovigilance obligations on the web, and to do so it will bring together a panel of speakers able to address all aspects of the issue: specialized lawyers, IT experts in online monitoring techniques, regional pharmacovigilance managers and coordinators and finally the pharmacovigilance managers at some of the major pharmaceutical multinationals present in Italy.
The seminar is aimed at personnel involved in pharmacovigilance processes and in the monitoring activity of the pharmaceutical company network: Pharmacovigilance Managers, Officers, QPPV, Quality Managers, Legal and Regulatory Affairs Managers, ICT managers, web marketing and communication representatives. These professionals can already register through the appropriate online form.
Memorandum. We recall that the Scientific Representatives of the drug based on art. 122 paragraph 6 of the Legislative Decree 219/06
– "should report to the scientific service referred to in article 126, on which they depend, and to the head of the pharmacovigilance service referred to in paragraph 4 of article 130, all information on the undesirable effects of medicines, attaching, where possible, copy of reporting cards used by the doctor pursuant to Title IX".
And in article 131 it is specified; that the Pharmacovigilance Manager ensures
– a) the establishment and operation of a system to ensure that information on all suspected adverse reactions communicated to company personnel and to medical-scientific informants, are collected, sorted and accessible in one place;
– b) that all information relating to the safety of medicinal products, subsequent to the act of authorisation, is rapidly brought to the attention of healthcare personnel, even through the contacts of the scientific information service of your company.