The evaluation of therapeutic equivalence between patent-covered drugs and others with expired patents, without the necessary scientific evidence, represents a clear weakening of the  patent protection. Speaks Fabrizio Greco, President of the Italian American Pharmaceutical Group (IAPG)
patent protection. Speaks Fabrizio Greco, President of the Italian American Pharmaceutical Group (IAPG)
ants – 5 July 2019 – by Fabrizio Greco
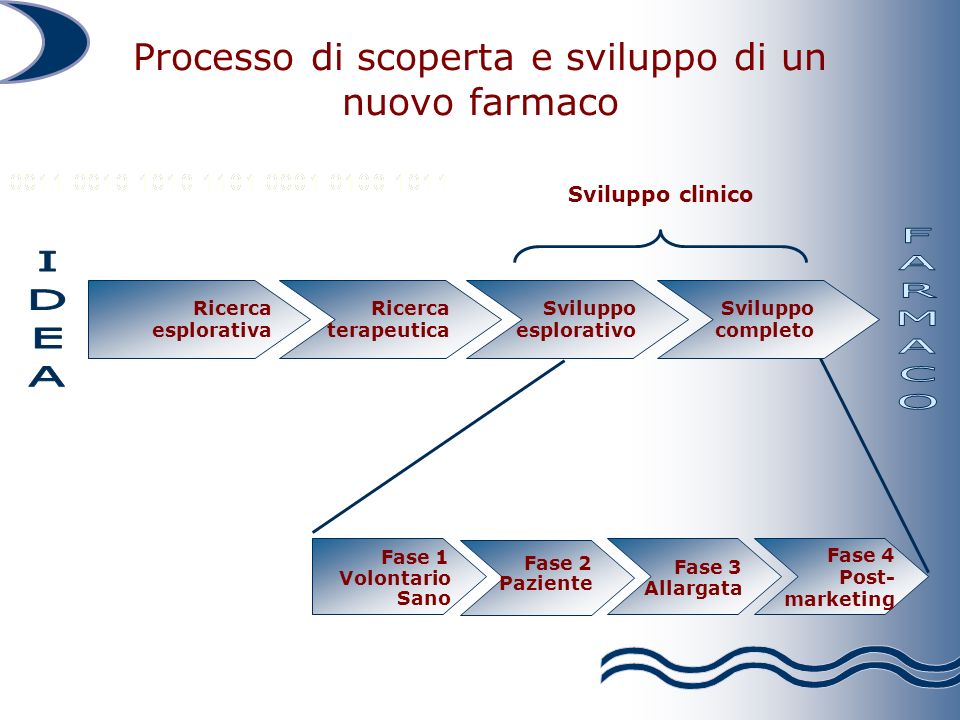
The patent for invention is a legal institution which guarantees the author the exclusive right of use of the object of the patent for twenty years from the filing date of the application, and is aimed at preventing third parties from producing, using, marketing it, sell or import it, except with the owner's consent. There are certain requirements that an industrial invention must possess in order to obtain patent protection, in detail: novelty, inventive activity, industrial applicability, lawfulness and repeatability.
The pharmaceutical sector is one of the fields in which the patent finds its maximum application because the research of new drugs is the founding activity of the sector and the aim is to allow the recovery of the huge resources necessary for the development of a new drug and to see recognized and valued the commitment and the risks involved in the various phases of the research. Furthermore, it should be considered that the pharmaceutical patent takes effect from the moment in which the exclusive right of use is requested which, as a rule, occurs from the early stages of starting the research. The period which elapses from the aforementioned research start-up phase until the release of the marketing authorization for a new drug and the definition of the price involves a considerable reduction in the time of use of the invention.
In practice, out of twenty years of patent protection, drug companies have about eight years of marketing left to recover their investments. In this context, what is the position  of Italy on the pharmaceutical patent? The value of the patent for scientific and technical progress in accordance with art. 9 of the Constitution would seem to represent a consolidated awareness in our country: already in 1978, in fact, the ban on the patenting of drugs was declared unconstitutional. Looking at more recent times, it is news in recent weeks that our country is engaged in a political-institutional initiative, supported across the board by all parties, to promote Italy's candidacy for the assignment to the city of Milan of the seat of the Unified European Patent Court, in particular the specialized section on pharmaceutical chemistry and life sciences disputes.
of Italy on the pharmaceutical patent? The value of the patent for scientific and technical progress in accordance with art. 9 of the Constitution would seem to represent a consolidated awareness in our country: already in 1978, in fact, the ban on the patenting of drugs was declared unconstitutional. Looking at more recent times, it is news in recent weeks that our country is engaged in a political-institutional initiative, supported across the board by all parties, to promote Italy's candidacy for the assignment to the city of Milan of the seat of the Unified European Patent Court, in particular the specialized section on pharmaceutical chemistry and life sciences disputes.
Elements, those mentioned, which would seem to support the aforementioned awareness on the part of Italy of the essential value of the patent for the development of the life sciences sector, of which the pharmaceutical sector is a founding pillar. A different, less enthusiastic interpretation emerges from a more careful analysis of the recent debates on the changes to sector policies announced by the government at the end of last year. In  particular, it clearly emerges how many of the measures under discussion risk nullifying the value of patent protection. Among these, the possibility for the Regions to request the Italian Medicines Agency (Aifa) to express its opinion on the existence of therapeutic equivalence between medicines containing different active ingredients, even those with expired patents, in order to proceed with the implementation of regional tenders for equivalence without explicitly requesting that any therapeutic equivalence be scientifically demonstrated for all types of patients.
particular, it clearly emerges how many of the measures under discussion risk nullifying the value of patent protection. Among these, the possibility for the Regions to request the Italian Medicines Agency (Aifa) to express its opinion on the existence of therapeutic equivalence between medicines containing different active ingredients, even those with expired patents, in order to proceed with the implementation of regional tenders for equivalence without explicitly requesting that any therapeutic equivalence be scientifically demonstrated for all types of patients.
Envisaging the evaluation of therapeutic equivalence between patent-covered drugs and others with expired patents, without the necessary scientific evidence, represents a clear weakening of patent protection, with the consequent disincentive for investments in research and development since such a scenario would not allow new drugs the expectation of finding an adequate return on the resources employed, so that they can be used for the discovery of future innovative therapies. Indeed, it is evident that the decision whether to proceed with the development of a new drug takes place many years before the generation of evidence whether or not such a drug brings added therapeutic value compared to the available treatment options.
If the objective risk of failure of the clinical research hypotheses were to be added to the uncertainty regarding the evaluation, not scientific but economistic, of the therapeutic equivalence between different active ingredients and regardless of patent coverage, the expectations of economic return of the investment, with consequent reduction of opportunities for the development of new therapies. Therefore, developing models which, in an attempt to obtain short-term economic benefits, jeopardize the value of patent protection, and therefore effectively discourage the search for new drugs, would therefore be counterproductive from a scientific and economic point of view and, above all, in perspective of patients, who expect innovative treatments from research for unmet therapeutic needs.
It is essential that innovation is supported by adequate measures, certain and stable rules, to ensure the progress of research for the benefit of people's health. Over the years, Italy has operated in full compliance with patent protection and, from 1951 to today, life expectancy has grown by a good 17 years, also stimulating research and strengthening the scientific, economic and industrial value of our country . In this perspective, the need emerges for sector governance which recognizes the scientific and therapeutic value of the discovery and which, through the protection of the patent, encourages the search for new therapies which make it possible to effectively address the health needs that are still not satisfied, as well as to reap the economic benefits deriving from the development of scientific knowledge and from a country in better health.