
Researchers in South Africa are monitoring the worrying increase of a new variant of the coronavirus (B.1.1.529) SARS-CoV-2 that causes COVID-19 which has become dominant in the country in two weeks. The variant carries a large number of the mutations found in other variants, including Delta, and spreads rapidly throughout South Africa. According to the scientific community, the variant B.1.1.529 would have originated in the body of an immunosuppressed patient because he was sick with AIDS. A terrain favorable to the evolution of the coronavirus.
 On November 6, the WHO Technical Advisory Group on the Evolution of the SARS-CoV-2 virus designated B.1.1.529 a “concern variant” and gave him the Omicron designation (skipping Nu or Xi, the next few letters of the Greek alphabet). The World Health Organization reserves the Omicron designation for “variants of interest“. The project GISID assigned it the identifier GR/4 4A and design Nextstrain assigned it the identifier 21K.
On November 6, the WHO Technical Advisory Group on the Evolution of the SARS-CoV-2 virus designated B.1.1.529 a “concern variant” and gave him the Omicron designation (skipping Nu or Xi, the next few letters of the Greek alphabet). The World Health Organization reserves the Omicron designation for “variants of interest“. The project GISID assigned it the identifier GR/4 4A and design Nextstrain assigned it the identifier 21K.
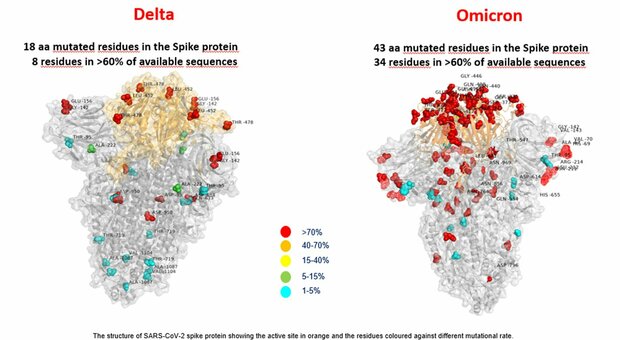
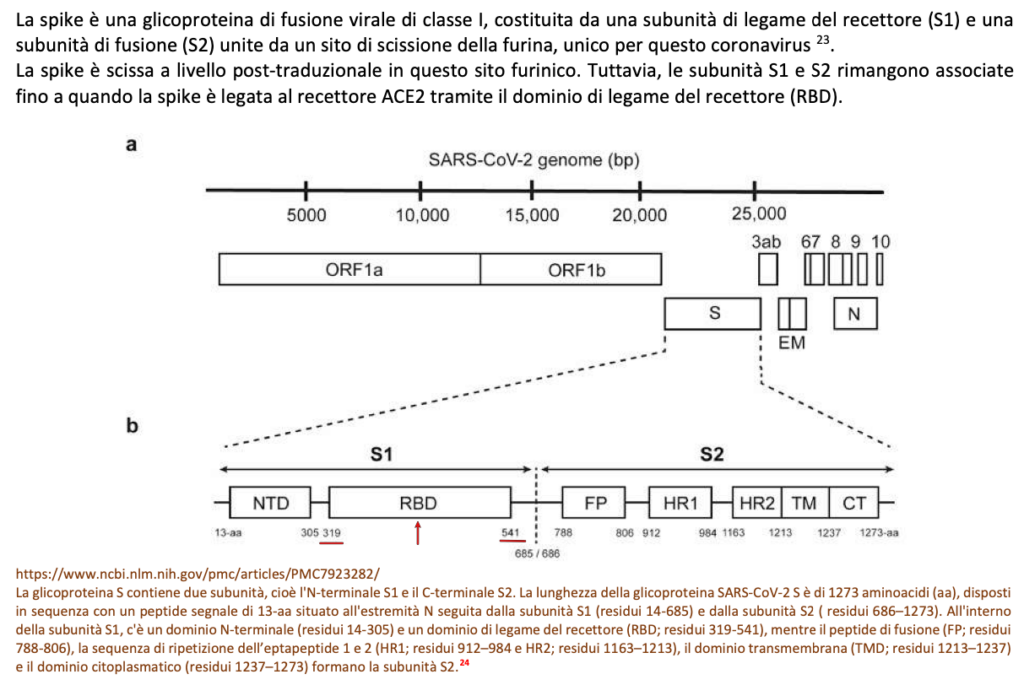
The variant has a large number of mutations, 32 of which affect the spike protein, the main antigenic target of antibodies generated by infections and by many widely administered vaccines. Many of these mutations had not been observed in other strains. The variant is characterized by 30 amino acid changes, three small deletions, and one small insertion in the protein 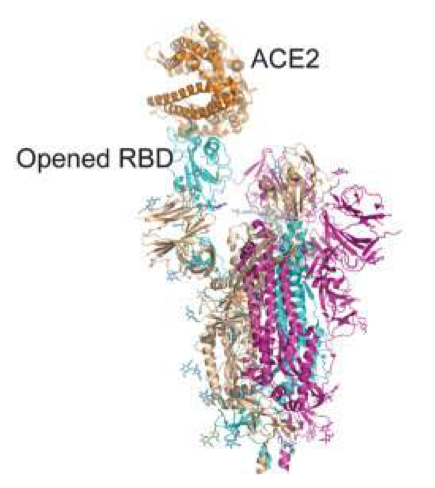 spikes compared to the original virus, of which 15 located in the receptor binding domain (RDB extension residues 319-541). It also carries a number of changes and deletions in other genomic regions. Of note, the variant has three mutations at the furin cleavage site. Furin cleavage site increases SARS-CoV-2 infectivity.
spikes compared to the original virus, of which 15 located in the receptor binding domain (RDB extension residues 319-541). It also carries a number of changes and deletions in other genomic regions. Of note, the variant has three mutations at the furin cleavage site. Furin cleavage site increases SARS-CoV-2 infectivity.
Sars-CoV-2 has a small polybasic sequence located laterally to the "spike". This extra "piece" is cut off by the cellular enzyme furin (protease). It is thanks to this cut that the "spike" can bind to a second receptor, neuropilin-1, which enhances the entry of the virus into the cells and the viral diffusion in the body, i.e. it allows the exposure of the fusion sequences and therefore allows the fusion of viral and cellular membranes, a step necessary for the virus to enter the cell.
Until yesterday it was classified among the variants to be monitored, now the WHO has reclassified it as "worrying".
Here are some excerpts from an article by Ewen Callaway published in the scientific journal "natures” on November 27th.
It will take a couple of weeks to understand if and to what extent the new Sars Cov 2 variant is able to escape the antibodies generated by the anti Covid vaccines, as well as the defenses due to the activation of the immune system's T cells, he said virologist Penny Moore, of the University of the Witwatersrand in Johannesburg. At the moment it is not even clear whether this variant is more transmissible than the Delta. The information available so far concerns some cases of reinfections and of cases in vaccinated individuals, he said again recalling that the vaccines used in South Africa are those of Johnson & Johnson, Pfizer-BioNtech or Oxford-AstraZeneca. However “at this stage it is too early to say anything”.
The situation is uncertain even for an infectious disease specialist Richard Lessells, from the University of KwaZulu-Natal in Durban: "There are many things we don't understand about this variant," he said at a press conference organized by South Africa's health department. "The profile of the mutation is worrying, but now we have to work to understand the characteristics of this variant in relation to the progress of the pandemic". The first information on the B.1.1.529 variant arrived thanks to genetic sequences obtained in Botswana.
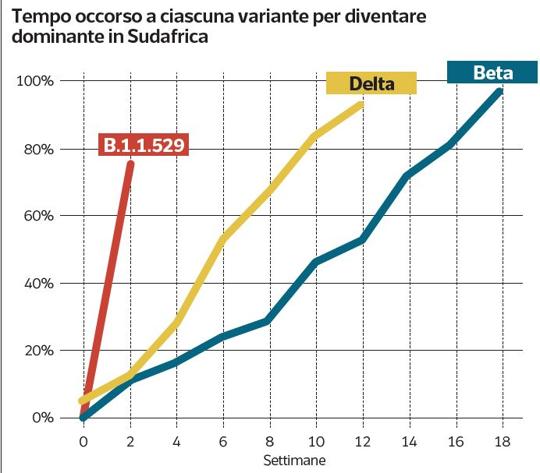 "Preliminary evidence suggests an increased risk of reinfection." The mutant has a large number of mutations, the experts explain, "some of which are worrying". “The number of cases of this variant appears to be on the rise in almost all provinces of South Africa“. WHO experts specify that the current diagnostics based on molecular tests "continue to detect" the Omicron variant. Several laboratories have indicated that with a widely used PCR test, one of the three target genes is missed and this test can then be used as a 'marker' to identify this variantpending sequencing confirmation. Using this approach this variant was detected at higher rates than previous infection spikes, suggesting that Omicron could have a growth advantage, in addition to the aforementioned higher risk of reinfection compared to other "concern variants". “Numerous studies are underway”, finally informs the WHO, and the Tag-Ve group of experts that follows the evolution of the virus “will continue to evaluate this variant. WHO will communicate the new findings to member states and the public as needed.
"Preliminary evidence suggests an increased risk of reinfection." The mutant has a large number of mutations, the experts explain, "some of which are worrying". “The number of cases of this variant appears to be on the rise in almost all provinces of South Africa“. WHO experts specify that the current diagnostics based on molecular tests "continue to detect" the Omicron variant. Several laboratories have indicated that with a widely used PCR test, one of the three target genes is missed and this test can then be used as a 'marker' to identify this variantpending sequencing confirmation. Using this approach this variant was detected at higher rates than previous infection spikes, suggesting that Omicron could have a growth advantage, in addition to the aforementioned higher risk of reinfection compared to other "concern variants". “Numerous studies are underway”, finally informs the WHO, and the Tag-Ve group of experts that follows the evolution of the virus “will continue to evaluate this variant. WHO will communicate the new findings to member states and the public as needed.
“A burning question is does it reduce the effectiveness of the vaccine, why does it have so many changes? “, says Aris Katzourakis, who studies the evolution of the virus at the University of Oxford, in the United Kingdom.
Modern communicated which is working rapidly to test the ability of the current vaccine dose to neutralize the Omicron variant and data is expected in the coming weeks.
Since the beginning of 2021, Moderna has advanced a global strategy to anticipate new “variants of concern“. This strategy includes three levels of response in the event that the currently licensed 50 µg booster dose of mRNA-1273 proves insufficient to boost waning immunity against the Omicron variant.
First, Moderna has already tested a higher dose booster of mRNA-1273 (100 µg) in healthy adults. Moderna completed dosing of 306 participants in a study on the safety and immunogenicity of this high-dose (100 µg) booster. The 100 µg dose of mRNA-1273 was also recently studied by the National Institutes of Health (NIH) in the United States and generally resulted in the highest neutralizing titers against previous strains of SARS-CoV-2. Moderna is working to rapidly test high-dose booster vaccines in neutralization trials to determine whether the 100 µg dose provides superior neutralizing protection against Omicron.
Second, Moderna is already clinically studying two multivalent booster candidates that have been designed to anticipate mutations such as those that have emerged in the Omicron variant. The first candidate (mRNA-1273.211) includes several mutations present in the Omicron variant that were also present in the Beta variant of interest. The Company has completed dosing in a potentially pivotal safety and immunogenicity study of mRNA-1273.211 at the 50 µg (N=300) and 100 µg (N=584) dose levels. Moderna will rapidly scale up testing of booster vaccines from completed and ongoing multivalent studies to determine whether these multivalent candidates can provide superior neutralizing protection against Omicron.
 Third, Moderna will rapidly advance an Omicron-specific booster candidate (mRNA-1273.529). This candidate is part of the company's strategy to advance specific candidates for a subset of variants of significant interest. In 2021 this has already included specific boosters for Beta and Delta. The company has repeatedly demonstrated the ability to advance new candidates to clinical trials in 60-90 days.
Third, Moderna will rapidly advance an Omicron-specific booster candidate (mRNA-1273.529). This candidate is part of the company's strategy to advance specific candidates for a subset of variants of significant interest. In 2021 this has already included specific boosters for Beta and Delta. The company has repeatedly demonstrated the ability to advance new candidates to clinical trials in 60-90 days.
“From the beginning, we have said that as we seek to defeat the pandemic, it is imperative to be proactive as the virus evolves. The mutations in the Omicron variant are worrying and for several days we have been moving as fast as possible to implement our strategy to address this variant,” said Stéphane Bancel, CEO of Moderna. “We have three lines of defense that we are pursuing in parallel: we have already evaluated a higher dose booster of mRNA-1273 (100 µg), secondly, we are already studying two multivalent booster candidates in the clinic which were designed to anticipate mutations such as those that have emerged in the Omicron variant and data are expected in the coming weeks, and third, we are rapidly advancing an Omicron-specific booster candidate (mRNA-1273.529).”
 The German laboratory BioNTech, an ally of Pfizer, also expects to have the first results of the studies available 'in two weeks at the latest' which will allow to determine whether the new variant detected in South Africa is able to escape the protection of the vaccine. A company spokesman made the announcement. 'Pfizer and BioNTech have been preparing for several months to adapt their vaccine in less than six weeks and to administer the first doses within 100 days', if a variant proves resistant.
The German laboratory BioNTech, an ally of Pfizer, also expects to have the first results of the studies available 'in two weeks at the latest' which will allow to determine whether the new variant detected in South Africa is able to escape the protection of the vaccine. A company spokesman made the announcement. 'Pfizer and BioNTech have been preparing for several months to adapt their vaccine in less than six weeks and to administer the first doses within 100 days', if a variant proves resistant.
According to the ISS vaccines determine the formation of an immune response against many fragments of the so-called Spike protein, the one, so to speak, produced by the virus to attach itself to cells and infect them. So even if there was a mutation in some fragments of the Spike protein it's unlikely that it would be enough to make the vaccine ineffective. From Istituto Mario Negri let it be known that there is no evidence - at the moment - that this variant is able to evade the vaccine. Prof. Shabir A. Madhi himself, one of the leading South African virologists and Executive Director of the National Institute for Communicable Disease, confirms that the vaccine maintains its level of protection. For this reason it is necessary to continue to promote vaccination in all countries and it is important to behave wisely and wait for more data on this new variant
The South African doctor Angelique Coetzee, President of the Doctors Association of South Africa and member of the Government Health Task Force, reports a mild symptomatology: «Fatigue, headache, itchy throat, slight cold. They didn't match the Delta ones we'd seen ten weeks earlier. We decided to test them because they were similar to a viral infection. No Omicron patients have been hospitalized so far. We have never experienced any serious effects. The interesting thing is that the patients with severe throat pain all tested negative." And he concludes: «You mustn't panic. If you experience this type of symptoms, like the ones described, for more than two days, get tested. Get vaccinated, wear a mask and don't stay in crowded places"
"To date (29/11/2021) no deaths related to the Omicron variant" of the coronavirus have been reported: the World Health Organization (WHO) stated in a technical document on the new variant of the coronavirus published today, the day on which holds emergency meeting of G7 health ministers.
Just scaremongering then? We will certainly know more in the coming weeks.
Related news: Heavily mutated coronavirus variant puts scientists on alert
WHO. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern
ISS. Prevalence and distribution of SARS-CoV-2 variants of public health interest in Italy
Innovative mRNA vaccine platforms
Bags in correction on Omicron, pharmaceuticals hold: excessive scare
Note:
The RBD receptor residues 319-541 site of 15 mutations in the omicron variant
The documents reported represent opinions, interpretations and reconstructions, even subjective ones and not necessarily shared by the editorial staff. Therefore, the responsibility of the statements lies with the author and/or whoever has provided us with the content. Our intention is to provide useful information for scientific representatives and to disseminate news of public interest. Naturally we invite readers to always deepen the subject matter, to consult more sources and we leave each of them the freedom of interpretation. The material found on the net, where the copyright wording and the rights of use have not already been declared on the site, has been deemed to be in the public domain in good faith. Some texts quoted or images included in this site are taken from the internet and, therefore, considered to be in the public domain; if their publication violates any copyright, please notify us via e-mail to provide for the consequent removal or modification.





