The new circular from the Ministry of Health was released on April 26, which updates the guidelines for home care of Covid patients from last November (Circular 30 November 2020).
 The document, drawn up by a working group made up of institutional, professional and scientific representatives, illustrates the methods of home management of the patient affected by COVID-19 by the general practitioner and the pediatrician of free choice on the basis of the knowledge available today. The guidelines also address caregivers, nurses and patients themselves.
The document, drawn up by a working group made up of institutional, professional and scientific representatives, illustrates the methods of home management of the patient affected by COVID-19 by the general practitioner and the pediatrician of free choice on the basis of the knowledge available today. The guidelines also address caregivers, nurses and patients themselves.
The recommendations refer to the pharmacological management of mild cases of Covid-19. In general, no therapy is indicated for people with these clinical characteristics, apart from a possible symptomatic supportive treatment.
Among the indications, the evaluation of patients to be referred to the reference structures for treatment with monoclonal antibodies is introduced, more accurate indications are given on the use of cortisone, the inappropriate uses of heparin are specified, the drugs not to be used are clearly indicated . Finally, in asymptomatic or paucisymptomatic subjects at home, the concept of "watchful waiting" is made explicit as active clinical surveillance, constant monitoring of the patient's vital parameters and clinical conditions.
In particular it is recommended to:
- do not modify, unless there is a compelling clinical reason, the chronic therapies in place for other pathologies (e.g. antihypertensive, hypolipidemic, hypoglycaemic, anticoagulant or antiplatelet therapies, psychotropic therapies)
- use a symptomatic treatment with paracetamol or NSAIDs in case of fever or joint or muscle pain, unless there is a clear contraindication to use, or other symptomatic drugs on clinical judgment
- do not routinely use corticosteroids; moreover, an early use of these drugs has proved to be useless if not harmful, as they are able to affect the development of an adequate immune response
- use heparin only in subjects immobilized due to the infection in progress
- avoid the empirical use of antibiotics; their possible use is to be reserved exclusively for cases in which the bacterial infection has been demonstrated by a microbiological examination and for those in which the clinical picture raises the well-founded suspicion of a bacterial overlap
- do not use hydroxychloroquine, the efficacy of which has not been confirmed in any of the randomized clinical trials conducted so far
- evaluate, in patients at risk of disease progression, the possibility of early treatment with monoclonal antibodies by the structures authorized to prescribe.
It should also be noted that, to date, there is no solid and incontrovertible evidence (i.e. deriving from clinical studies 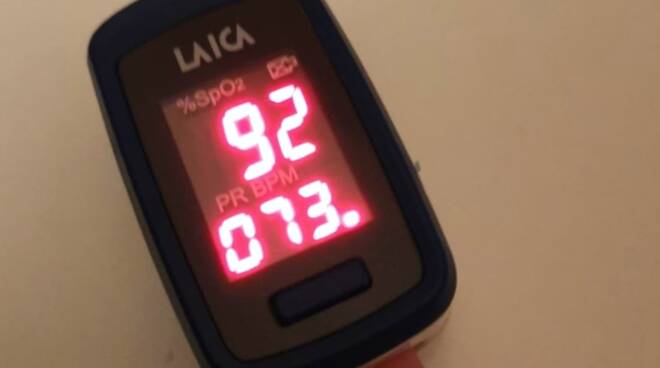 controlled) efficacy of vitamin supplements and food supplements (such as vitamins, including vitamin D, lactoferrin, quercitin), the use of which for this indication is, therefore, not recommended.
controlled) efficacy of vitamin supplements and food supplements (such as vitamins, including vitamin D, lactoferrin, quercitin), the use of which for this indication is, therefore, not recommended.
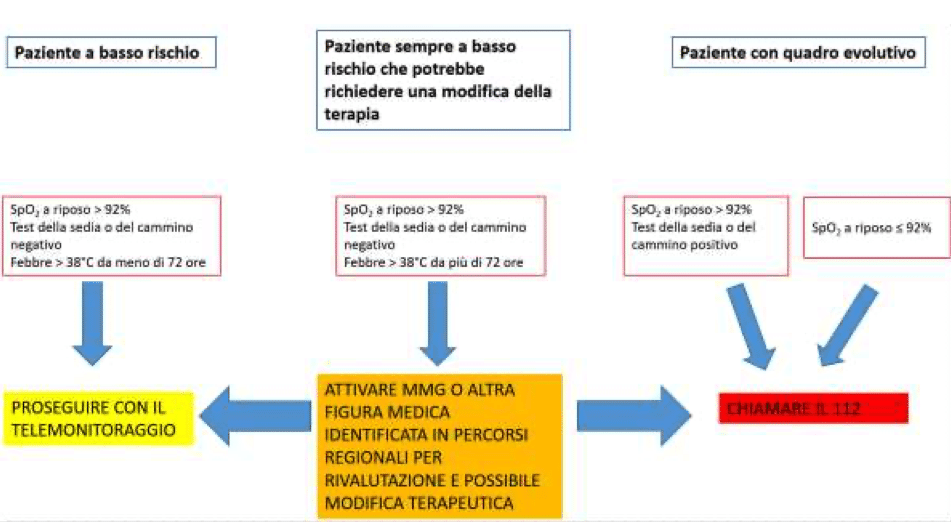
Based on the analysis of the scientific literature available to date and on the basis of the technical characteristics of the commercially available pulse oximeters for extra-hospital use, it is considered to consider the safety threshold value for a Covid-19 patient at home 92% oxygen saturation.
If oxygen saturation drops below 92%, evaluate hospitalization and oxygen therapy at home.
Given the constant evolution of knowledge on the infection, the course of the Covid-19 disease and the therapeutic possibilities, the document will be periodically updated, in order to bring the indications into line with international clinical practice, on the basis of emerging scientific knowledge.
Laws
- Ministry of Health Circular 26 April 2021 – Home management of patients infected with SARS-CoV-2, updated on April 26, 2021
Ministry of Health - update 28 April 2021