L![]() Italy has until 2020 to replace the current adhesive labels on medicines with plates printed on optically readable cases: even if the electronic prescription is advancing, the Italian model is a guarantee against counterfeiting and Europe allows us to keep it longer. He explained it Vito DeFilippo (in the photo) Undersecretary of Health a Cosimo Latronico Forza Italia parliamentarian who in a question recalled how the Italian adhesive label system is expected not only from the dematerialisation of recipes from European directive 62 of 2011.
Italy has until 2020 to replace the current adhesive labels on medicines with plates printed on optically readable cases: even if the electronic prescription is advancing, the Italian model is a guarantee against counterfeiting and Europe allows us to keep it longer. He explained it Vito DeFilippo (in the photo) Undersecretary of Health a Cosimo Latronico Forza Italia parliamentarian who in a question recalled how the Italian adhesive label system is expected not only from the dematerialisation of recipes from European directive 62 of 2011.
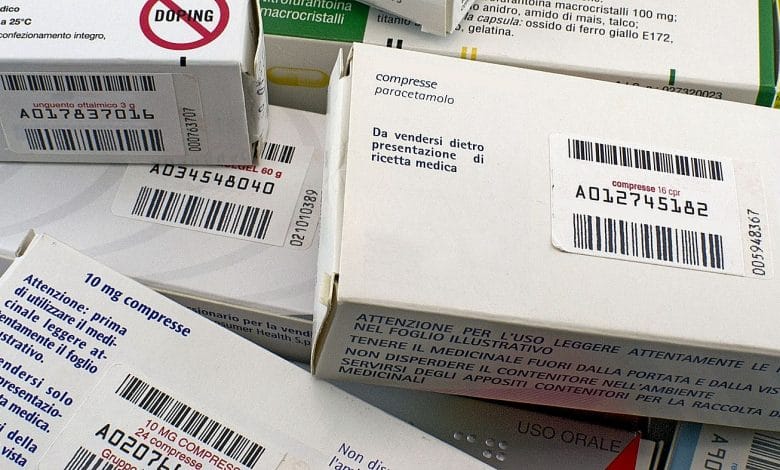
«The new European model – underlines Latronico to Farmacista33 – provides for a coding printed on the drug case, the adoption of which would save about 50% (it would cost around 11 euros per 1000 packs ed) on current costs and would give greater guarantees of avoiding illegal trafficking the introduction into the Italian market of untraced batches of medicines». Furthermore, as the Ministry of Health recalls on its website regarding the Project, the current adhesive stamps present on all the medicines sold have done their job very well. Their data, recorded by the company, then by the wholesaler, then by the distributor up to the pharmacist during the dispensing phase, allows the Ministry's database to know "at any time where a box of pills or a bottle of syrup is , in the hospital, pharmacy or even at a patient's home”. However, this mechanism does not work on the dematerialized recipe, which by 2016 will also be widespread in all Italian regions and is already widespread in Trentino, Val d'Aosta, Veneto, Sicily and Basilicata.
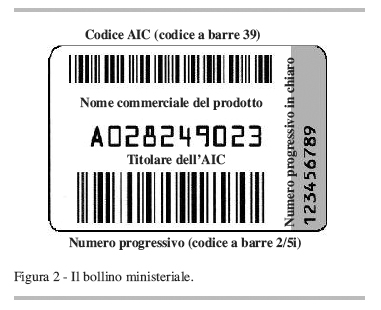
 But how do you put the sticker today if the recipe is no longer there? «Depending on the regions, either you attach it to the memo printed by the family doctor and issued to the patient, or as happens in Trento where the memo is not used, the labels classified in separate registers are delivered to the ASL it scans and stores in memory and then cross-references with online prescription data,” he explains John Petrosillo president of Federfarma Bergamo and national secretary of Federfarma. In 2014, a ministerial decree took a first step towards the computerization of the label by introducing the Datamatrix code to be printed on the package, readable with an optical device. «The Datamatrix coded label - recalls Petrosillo - can contain more information than paper stamps: the latter the Aic code of the specialty and the progressive number of the package, in the 140 characters of the Datamatrix you should also read the lot and the expiry date.
But how do you put the sticker today if the recipe is no longer there? «Depending on the regions, either you attach it to the memo printed by the family doctor and issued to the patient, or as happens in Trento where the memo is not used, the labels classified in separate registers are delivered to the ASL it scans and stores in memory and then cross-references with online prescription data,” he explains John Petrosillo president of Federfarma Bergamo and national secretary of Federfarma. In 2014, a ministerial decree took a first step towards the computerization of the label by introducing the Datamatrix code to be printed on the package, readable with an optical device. «The Datamatrix coded label - recalls Petrosillo - can contain more information than paper stamps: the latter the Aic code of the specialty and the progressive number of the package, in the 140 characters of the Datamatrix you should also read the lot and the expiry date.
 There is a roadmap for adopting scanners in pharmacies but we are at the beginning and manufacturers are now inserting the same data as the stamps». In the meantime, the European Union has granted Italy an extension for the mint stamps: six years starting from the transposition of the directive which took place in 2014. «Europe is thinking for now of tracing only drugs at risk of counterfeiting: it only life-saving but also the most expensive and best-selling on the web. In order to have the same degree of security as the paper sticker system - reflects Petrosillo - it will be necessary to prepare a repository where not only the data of class A medicines but also at least the most used products in class C will flow. Only when we have a container in capable of potentially accommodating all or almost all of the current stickers, it will be possible to leap in quality to the "dematerialized" plate.
There is a roadmap for adopting scanners in pharmacies but we are at the beginning and manufacturers are now inserting the same data as the stamps». In the meantime, the European Union has granted Italy an extension for the mint stamps: six years starting from the transposition of the directive which took place in 2014. «Europe is thinking for now of tracing only drugs at risk of counterfeiting: it only life-saving but also the most expensive and best-selling on the web. In order to have the same degree of security as the paper sticker system - reflects Petrosillo - it will be necessary to prepare a repository where not only the data of class A medicines but also at least the most used products in class C will flow. Only when we have a container in capable of potentially accommodating all or almost all of the current stickers, it will be possible to leap in quality to the "dematerialized" plate.