Priority by age and category has been abolished, and more and more young people are asking for the Anglo-Swedish vaccine. Why? And how is its distribution going?
The fall of the last limitation has unleashed a "race" to AstraZeneca, in Germany. These are the facts: at the beginning of the month, by decision of the federal government and the Laender, it was in the country abolished the priority rule  by age groups and categories in the administration of vaccines. The consequences of this choice are partly surprising: not only has the number of people who presented themselves to receive the vaccine grown exponentially, and this could have been predicted. But the specific requests for AstraZeneca have increased dramatically: unpredictable, if you think about it skepticism which has been revolving around the Anglo-Swedish vaccine for months, and if we think that, in March, Germany was among the first in Europe to suspend its administration to verify its links with some rare cases of thrombosis.
by age groups and categories in the administration of vaccines. The consequences of this choice are partly surprising: not only has the number of people who presented themselves to receive the vaccine grown exponentially, and this could have been predicted. But the specific requests for AstraZeneca have increased dramatically: unpredictable, if you think about it skepticism which has been revolving around the Anglo-Swedish vaccine for months, and if we think that, in March, Germany was among the first in Europe to suspend its administration to verify its links with some rare cases of thrombosis.
As reported by the Rheinische Post, newspaper of Dusseldorf, however, the German pharmacist association launches an alarm: “AstraZeneca is now in such great demand that the quantities ordered may no longer be sufficient“, said the president of the association Thomas Preis. According to the weekly der Spiegel, then, the current "success" of AstraZeneca is given by being able to shorten i recall times to four weeks, instead of twelve, and this would have made the vaccine more popular among young people, eager to be immunized for the start of summer and reopening. There are not a few cases of medical studies literally stormed with the specific request to receive AstraZeneca.
WHAT HAPPENS WITH ASTRAZENECA
AstraZeneca, you will recall, was not at the center of the public debate only for the alleged links with cases of thrombosis, later classified by the EMA as rare side effects of the vaccine (“but the benefits outweigh the risks”). The problems with the pharmaceutical company – who manufactures with Oxford – started earlier, with i delays almost starting from the first day in dose deliveries. By failing to comply with the contractual agreements with theEuropean Union. With the slowdowns in the production itself of vaccines and with accusations of favoring other buyers over the EU.
 Between the company and Europe, therefore, ended up in court. On April 23, the European Commission launched a legal action against the pharmaceutical company for the violation of the “advance purchase agreement”of vaccines against Covid. This was stated by the spokesman for Health of the EU executive, Stefan de Keersmaecker, who underlined how the lawsuit was initiated for the non-compliance with contractual terms, and for the company's failure to put in place a "reliable strategy to ensure timely delivery of doses". All 27 members of the block gave the go ahead.
Between the company and Europe, therefore, ended up in court. On April 23, the European Commission launched a legal action against the pharmaceutical company for the violation of the “advance purchase agreement”of vaccines against Covid. This was stated by the spokesman for Health of the EU executive, Stefan de Keersmaecker, who underlined how the lawsuit was initiated for the non-compliance with contractual terms, and for the company's failure to put in place a "reliable strategy to ensure timely delivery of doses". All 27 members of the block gave the go ahead.
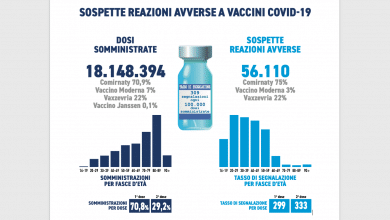
The last 24 million doses of AstraZeneca should arrive in Italy by the end of September. At the moment, just over 6.6 million have been delivered in our country, against almost 20 of Pfizer-Biontech.
Related news: The peace vaccine for poor countries Oxford-Astrazeneca deserve the Nobel